March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 3. Bonding Weaker Than Covalent
3.B. π–π Interactions
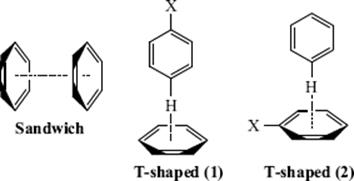
Many theoretical and experimental studies clearly show the importance of π–π interactions,77 which are fundamental to many supramolecular organization and recognition processes.78 Perhaps the simplest prototype of aromatic π–π interactions is the benzene dimer.79 Within dimeric aryl systems such as this, possible π–π interactions are the sandwich and T-shaped interactions shown. It has been shown that all substituted sandwich dimers bind more strongly than a benzene dimer, whereas the T-shaped configurations bind more or less favorably depending on the substituent.80 Electrostatic, dispersion, induction, and exchange-repulsion contributions are all significant to the overall binding energies.80
The π electrons of aromatic rings can interact with charged species, yielding strong cation–π interactions that are dominated by electrostatic and polarization effects.81 An interaction with CH units is also possible. For CH–π interactions in both alkyl- and aryl-based model systems, dispersion effects dominate the interaction, but the electrostatics term is also relevant for aryl CH–π interactions.82
Detection of π–π interactions has largely relied on NMR based techniques (e.g., chemical shifts variations),83 and Nuclear Overhauser Effect Spectroscopy (NOESY) or Rotating-Frame NOE Spectroscopy (ROESY).84 Diffusion-ordered NMR spectroscopy (DOSY) has also been used to detect π–π stacked complexes.85