March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 3. Bonding Weaker Than Covalent
3.C. Addition Compounds
When the reaction of two compounds results in a product that contains all the mass of the two compounds, the product is called an addition compound. There are several kinds. The remainder of this chapter will discuss addition compounds in which the molecules of the starting materials remain more or less intact and weak bonds hold two or more molecules together. There are four broad classes: electron donor–acceptor complexes, complexes formed by crown ethers and similar compounds, inclusion compounds, and catenanes.
3.C.i. Electron Donor–Acceptor Complexes86
In electron donor–acceptor (EDA) complexes,87 there is always a donor and an acceptor molecule. The donor may donate an unshared pair (an n donor) or a pair of electrons in a π orbital of a double bond or aromatic system (a π donor). The electronic spectrum constitutes a good test for the presence of an EDA complex. These complexes generally exhibit a spectrum (called a charge-transfer spectrum) that is not the same as the sum of the spectra of the two individual molecules.88 Because the first excited state of the complex is relatively close in energy to the ground state, there is usually a peak in the visible or near-UV region, and EDA complexes are often colored. Many EDA complexes are unstable and exist only in solutions in equilibrium with their components, but others are stable solids. In most EDA complexes, the donor and acceptor molecules are present in an integral ratio, most often 1:1, but complexes with nonintegral ratios are also known. There are several types of acceptor molecules; complexes formed by two of them will be discussed.
1. Complexes in which the Acceptor is a Metal Ion and the Donor is an Alkene or an Aromatic Ring.
The n donors do not give EDA complexes with metal ions, but form covalent bonds instead).89 Many metal ions form complexes with alkenes, dienes (usually conjugated, but not always), alkynes, and aromatic rings that are often stable solids. The donor (or ligand) molecules in these complexes are classified by the prefix hapto90 and/or the descriptor ηn (the Greek letter eta), where n indicates how many atoms the ligand uses to bond with the metal.91
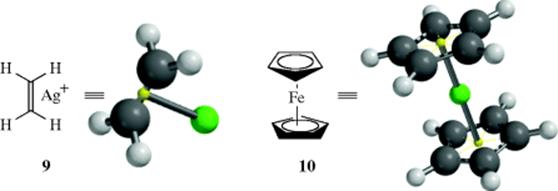
The generally accepted picture of the bonding in these complexes,92 first proposed by Dewar,93 can be illustrated by the ethylene complex with silver (9), in which the alkene unit forms an η2-complex with the silver ion (the alkene functions as a 2-electron donating ligand to the metal). There is evidence of π-complexation of Na+ by C=C.94 In the case of the silver complex, the bond is not from one atom of the C=C unit to the silver ion, but from the π center (two electrons are transferred from the alkene to the metal ion). Ethene has two π-electrons and is a dihapto or η2 ligand, as are other simple alkenes. Similarly, benzene has six π electrons and is a hexahapto or η6ligand. Ferrocene (10) is an example of a metallocene, with two cyclopentadienyl ligands (each is a five-electron donor or an η5 ligand), and ferrocene is properly called bis(η5-cyclopentadienyl)iron(II). This system can be extended to compounds in which only a single σ bond connects the organic group to the metal, (e.g., C6H5–Li a monohapto or η1 ligand), and to complexes in which the organic group is an ion, (π-allyl complexes, e.g., 11), in which the allyl ligand is trihapto or η3. Note that in a compound such as allyllithium, where a σ bond connects the carbon to the metal, the allyl group is referred to as monohapto or η1.
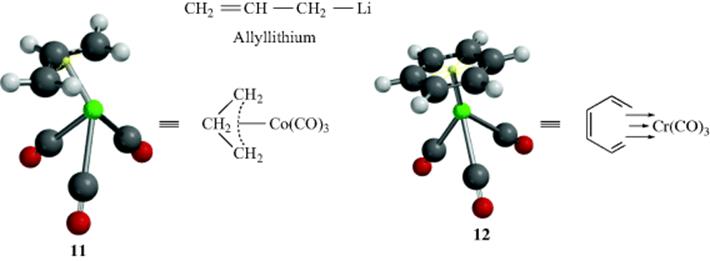
As mentioned, benzene is an η6 ligand that forms complexes with silver and other metals.95 When the metal involved has a coordination number > 1, more than one donor molecule (ligand) participates. The CO group is a common ligand (a two-electron donating or η2 ligand), and in metal complexes the CO group is classified as a metal carbonyl. Benzenechromium tricarbonyl (12) is a stable compound96 that illustrates both benzene and carbonyl ligands. Three arrows are shown to represent the six-electron donation (an η6 ligand), but the accompanying model gives a clearer picture of the bonding. Cyclooctatetraene is an eight-electron donating or η8 ligand that also forms complexes with metals. Metallocenes (e.g., 10) may be considered a special case of this type of complex, although the bonding in metallocenes is much stronger.
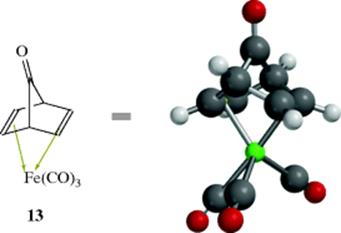
In a number of cases, alkenes that are too unstable to be isolated as discreet molecules have been isolated in the form of their metal complexes. An example is norbornadienone, which was isolated as its iron–tricarbonyl complex (13),97 where the norbornadiene unit is an η4 ligand, and each of the carbonyl units are η2 ligands. The free dienone spontaneously decomposes to carbon monoxide and benzene (see Reaction 17–28).
2. Complexes in Which the Acceptor Is an Organic Molecule.
Picric acid (2,4,6-trinitrophenol) and similar polynitro compounds are the most important of these.98 Picric acid forms addition compounds with many aromatic hydrocarbons, aromatic amines, aliphatic amines, alkenes, and other compounds. These addition compounds are usually solids that have definite melting points and have been used as derivatives of the compounds in question. They are called picrates, and are addition compounds and not salts of picric acids. Unfortunately, the actual salts of picric acid are also called picrates. Similar complexes are formed between phenols and quinones (quinhydrones).99 Alkenes that contain electron-withdrawing substituents also act as acceptor molecules, as do carbon tetrahalides100 and certain anhydrides.101 A particularly strong alkene acceptor is tetracyanoethylene.102
The bonding in these cases is more difficult to explain than in the previous case, and indeed no truly satisfactory explanation is available.103 The difficulty is that the donor has a pair of electrons to contribute (both n donors and π donors are found here), but the acceptor does not have a vacant orbital. Simple attraction of the dipole–induced-dipole type accounts for some of the bonding,104 but is too weak to explain the bonding in all cases.105Nitromethane, with about the same dipole moment as nitrobenzene, is an example of a molecule that forms much weaker complexes. Some other type of bonding clearly must also be present in many EDA complexes. The exact nature of this bonding, called charge-transfer bonding, is not well understood, but it presumably involves some kind of donor–acceptor interaction.
3.C.ii Crown Ether Complexes and Cryptates106
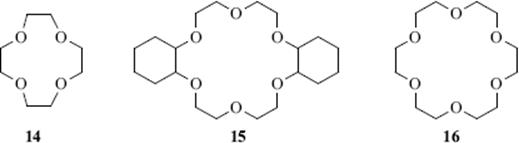
Crown ethers are large-ring compounds that contain several oxygen atoms, usually in a regular pattern. Examples are 12-crown-4 (14; where 12 is the size of the ring and 4 represents the number of coordinating atoms, here oxygen),107 dicyclohexano-18-crown-6 (15), and 15-crown-5 (16). These compounds have the property108 of forming complexes with positive ions, generally metallic ions (though not usually ions of transition metals) or ammonium and substituted ammonium ions.109 The crown ether is called the host and the ion is the guest. In most cases, the ions are held tightly in the center of the cavity.110 Each crown ether binds different ions, depending on the size of the cavity. For example, 14 binds Li+111, but not K+,112 while 15 binds K+ but not Li+.113 Similarly, 15 binds Hg2+, but not Cd2+ or Zn2+, and Sr2+ but not Ca2+.114 18-Crown-5 binds alkali and ammonium cations >1000 times weaker than 18-crown-6, presumably because the larger 18-crown-6 cavity involves more hydrogen bonds.115 The complexes can frequently be prepared as well-defined sharp-melting solids.
Apart from their obvious utility in separating mixtures of cations,116 crown ethers are widely used in organic synthesis (see the discussion on Sec. 10.G.v). Chiral crown ethers have been used for the resolution of racemic mixtures (Sec. 4.A). Although crown ethers are most frequently used to complex cations, amines, phenols, and other neutral molecules have also been complexed117 (see Sec. 4.L for the complexing of anions).118 Macrocycles containing nitrogen (azacrown ethers) or sulfur atoms (thiacrown ethers)119 (e.g., 17 and 18),120 have complexing properties similar to other crown ethers, as do mixed-heteroatom crown ethers (e.g., 19,12120,122 or 21123).
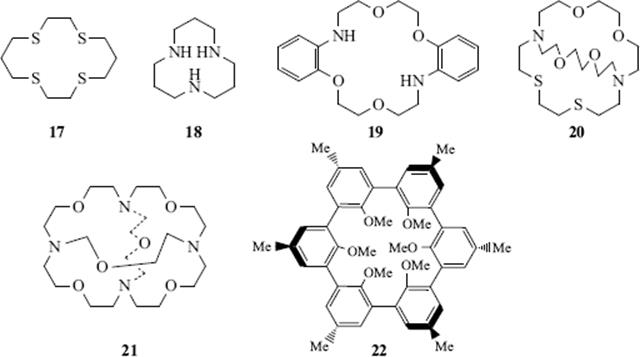
Bicyclic molecules (e.g., 20) can surround the enclosed ion in 3D, binding it even more tightly than the monocyclic crown ethers. Bicyclics and cycles of higher order124 are called cryptands and the complexes formed are called cryptates (monocyclic compounds are sometimes called cryptands). When the molecule contains a cavity that can accommodate a guest molecule, usually through hydrogen-bonding interactions, it is sometimes called a cavitand.125The tricyclic cryptand 21 has 10 binding sites and a spherical cavity.98 Another molecule with a spherical cavity (though not a cryptand) is 22, which complexes Li+ and Na+ (preferentially Na+), but not K+, Mg2+, or Ca2+.126Molecules such as these, whose cavities can be occupied only by spherical entities, have been called spherands.83 Other types are calixarenes127 (e.g., 23).128 Spherand-type calixarenes are known.129 There is significant hydrogen bonding involving the phenolic OH units in [4]calixarenes, but this diminishes as the size of the cavity increases in larger ring calixarenes.130 There are also calix[6]arenes131 that have been shown to have conformational isomers (Sec. 4.O) in equilibrium (cone vs alternate) that can sometimes be isolated:132 calix[8]arenes,133 azacalixarenes,134 homooxacalixarenes,135 and calix[9–20]arenes.136 Note that substitution of the unoccupied “meta” positions immobilizes calix[4]arenes and the conformational mobility (Sec. 4.O.iv) in calix[8]arenes is substantially diminished.137 Amide-bridged calix[4]arenes138 calix[4]azulene,139 and quinone-bridged calix[6]arenes.140 are known, and diammoniumcalix[4]arene has been prepared.141 Enantiopure calix[4]resorcinarene derivatives are known,142 and water-soluble calix[4]arenes have been prepared.143 There are also a variety of calix[n]crown ethers,144 some of which are cryptands145 and there is evidence for formation of a calix[4]arene-proton complex.146
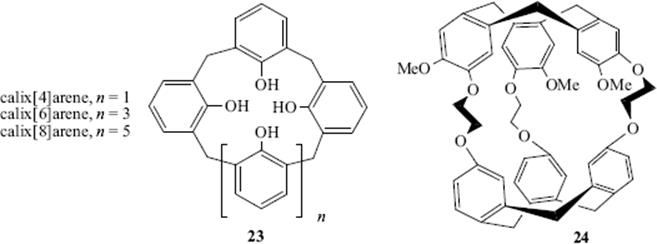
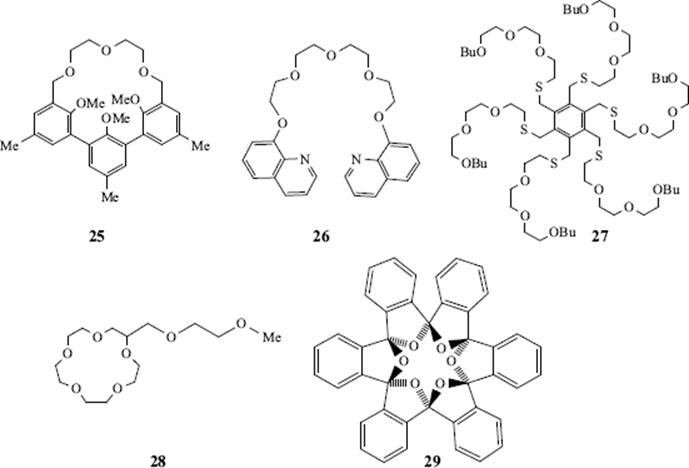
Other molecules include cryptophanes (e.g., 24),147 hemispherands (an example is 25148), and podands.149 The last-named are host compounds in which two or more arms come out of a central structure. Examples are 26150 and 27151 and the latter molecule binds simple cations (e.g., Na+, K+, and Ca2+. Lariat ethers152 are compounds containing a crown ether ring with one or more side chains that can also serve as ligands, (e.g., 28).153 There is also a class of ortho cyclophanes that are crown ethers (see 29) and have been given the name starands.154
The bonding in these complexes is the result of ion-dipole attractions between the heteroatoms and the positive ions. The parameters of the host–guest interactions can sometimes be measured by NMR.155
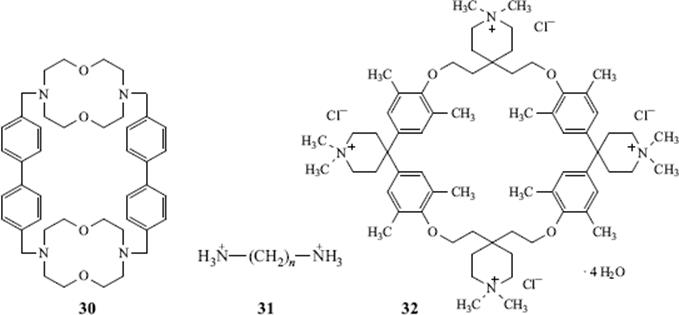
It has been implied that the ability of these host molecules to bind guests is often very specific, often linked to the hydrogen-bonding ability of the host,156 enabling the host to pull just one molecule or ion out of a mixture. This is called molecular recognition.157 In general, cryptands, with their well-defined 3D cavities, are better for this than monocyclic crown ethers or ether derivatives. An example is the host (30), which selectively binds the dication 31 (n= 5) rather than 31 (n = 4), and 31 (n = 6) rather than 31 (n = 7).158 The host 32, which is water-soluble, forms 1:1 complexes with neutral aromatic hydrocarbons (e.g., pyrene and fluoranthene), and even (though more weakly) with biphenyl and naphthalene, I is also capable of transporting them through an aqueous phase.159
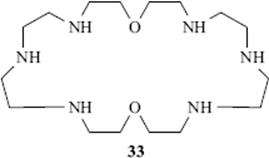
Of course, it has long been known that molecular recognition is very important in biochemistry. The action of enzymes and various other biological molecules is extremely specific because these molecules also have host cavities that are able to recognize only one or a few particular types of guest molecules. Now, organic chemists can synthesize non-natural hosts that can also perform crude (compared to biological molecules) molecular recognition. The macrocycle 33 has been used as a catalyst, for the hydrolysis of acetyl phosphate and the synthesis of pyrophosphate.160
No matter what type of host, the strongest attractions occur when combination with the guest causes the smallest amount of distortion of the host.161 That is, a fully preorganized host will bind better than a host whose molecular shape must change in order to accommodate the guest.
3.C.iii Inclusion Compounds
This type of addition compound is different from either the EDA complexes or the crown ether type of complexes previously discussed. Here, the host forms a crystal lattice that has spaces large enough for the guest to fit into. van der Waals forces constitute the only bonding between the host and the guest. There are two main types, depending on the shape of the space.162 The spaces in inclusion compounds, are in the shape of long tunnels or channels, while the other type, often called clathrate,163 or cage compounds, have spaces that are completely enclosed. In both types, the guest molecule must fit into the space and potential guests that are too large or too small will not go into the lattice, so that the addition compound will not form. Such structures need not be restricted to large molecules. Indeed, the structure and stability of the hydrogen clathrate hydrate with cyclohexanone is known.164
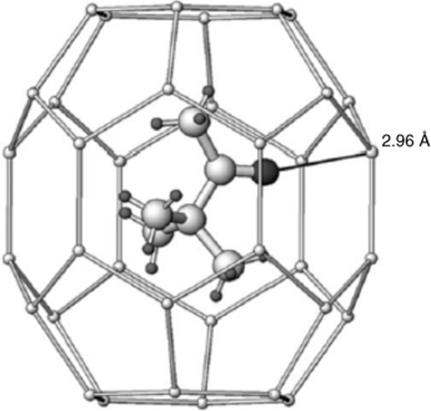
Several important host molecules are known, and inclusion compounds include small molecules (e.g., urea).165 Hydrogen sulfide forms hexagonal clathrate hydrate cages, and a guest molecule (e.g., pinacolone), may be present, as shown in Fig. 3.1.166 Commonly, van der Waals forces between the host and the guest, while small, are essential to the stability of the structure. Which molecules can be a guest is usually dependent on their shapes and sizes and not necessarily on any electronic or chemical effects. For example, octane and 1-bromooctane are suitable guests for urea, but 2-bromooctane, 2-methylheptane, and 2-methyloctane are not. Also, both dibutyl maleate and dibutyl fumarate are guests; neither diethyl maleate nor diethyl fumarate is a guest, but dipropyl fumarate is a guest and dipropyl maleate is not.167 In these complexes, there is usually no integral molar ratio (though by chance there may be). For example, the octane/urea ratio is 1:6.73.168 A deuterium quadrupole echo spectroscopy study of a urea complex showed that the urea molecules do not remain rigid, but undergo 180° flips about the C=O axis at the rate of >106 s–1 at 30 °C.169
Fig. 3.1 X-ray strucutre of pinacolone in a H2S hexagonal clathrate hydrate cage molecule at 100 K.166 [Reprinted with permission from Alavi, S.; Udachin, K.; Ripmeester, J.A. Chem. Eur. J. 2010, 16, 1017, Wiley–VCH Verlag GmbH & Co. KGaA, Weinheim. Copyright © 2010 by Wiley–VCH Verlag.]
The complexes are solids, but are not useful as derivatives, since they melt, with decomposition of the complex at the melting point of urea. They are useful, however, in separating isomers that would be quite difficult to separate otherwise. Thiourea also forms inclusion compounds though with channels of larger diameter, so that n-alkanes cannot be guests but, (e.g., 2-bromooctane, cyclohexane, and chloroform readily fit).
Hydroquinone is a useful host for clathrates.170 Three molecules, held together by hydrogen bonding, make a cage in which fits one molecule of guest. Typical guests are methanol (but not ethanol), sulfur dioxide, carbon dioxide, and argon (but not neon). One important use is the isolation of anhydrous hydrazine as complex.171 Anhydrous hydrazine is highly explosive, so preparation by distillation of aq hydrazine solutions is both difficult and dangerous. The inclusion complex can be readily isolated and reactions done in the solid state (e.g., the reaction with esters to give hydrazides, Reaction 16–75).172 In contrast to the inclusion compounds, the crystal lattices here can exist partially empty. Another host is water. Usually six molecules of water form the cage and many guest molecules, among them Cl2, propane, and methyl iodide, can fit. The water clathrates (see Fig. 3.1), which are solids, can normally be kept only at low temperatures; at room temperature, they decompose.171 Methane hydrate, which is a promising energy source that exists is vast quantities at in the seabed of various oceans,173 is an example of this type of clathrate. Another inorganic host is sodium chloride (and some other alkali halides), which can encapsulate organic molecules (e.g., benzene, naphthalene, and diphenylmethane).174
Among other hosts175 for inclusion and/or clathrate compounds are deoxycholic acid,176 cholic acid,177 anthracene compounds, (e.g., 34),178 dibenzo-24-crown-8,179 and the compound 35, which has been called a carcerand.180When carcerand-type molecules trap ions or other molecules (called guests), the resulting complex is called a carciplex.181 It has been shown that in some cases, the motion of the guest within the carciplex is restricted.182
3.C.iv Cyclodextrins
There is one type of host that can form both channel and cage complexes. This type is called cyclodextrins or cycloamyloses.183 The host molecules are made up of six, seven, or eight glucose units connected in a large ring, called, respectively, α-, β-, or γ-cyclodextrin (Fig. 3.2 shows the β or seven-membered ring compound). The three molecules are in the shape of hollow truncated cones (Fig. 3.3) with primary OH groups projecting from the narrow side of the cones and secondary OH group from the wide side. As expected for carbohydrate molecules, all of them are soluble in water and the cavities normally fill with water molecules held in place by hydrogen bonds (6, 12, and 17 H2O molecules for the α, β, and γ forms, respectively), but the insides of the cones are less polar than the outsides, so that nonpolar organic molecules readily displace the water. The polarity of such cavities has been probed by a chemical reaction: the solvolysis of benzoyl halides Reaction (16–57).184 Thus the cyclodextrins form 1:1 cage complexes with many guests, ranging in size from the noble gases to large organic molecules. A guest molecule must not be too large or it will not fit, though many stable complexes are known in which one end of the guest molecule protrudes from the cavity (Fig. 3.4). On the other hand, if the guest is too small, it may go through the bottom hole (though some small polar molecules, e.g., methanol, do form complexes in which the cavity also contains some water molecules). Since the cavities of the three cyclodextrins are of different sizes (Fig. 3.3), a large variety of guests can be accommodated. Since cyclodextrins are nontoxic (they are actually small starch molecules), they are now used industrially to encapsulate foods and drugs.187
Fig. 3.2 β-Cyclodextrin.
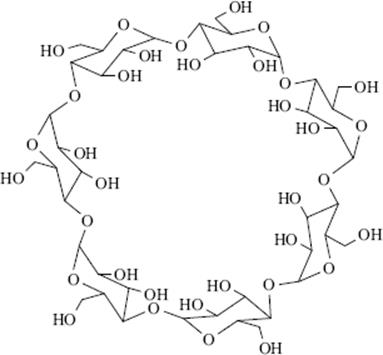
Fig. 3.3 Shape and dimensions of the α-, β-, and γ-cyclodextrin molecules.185
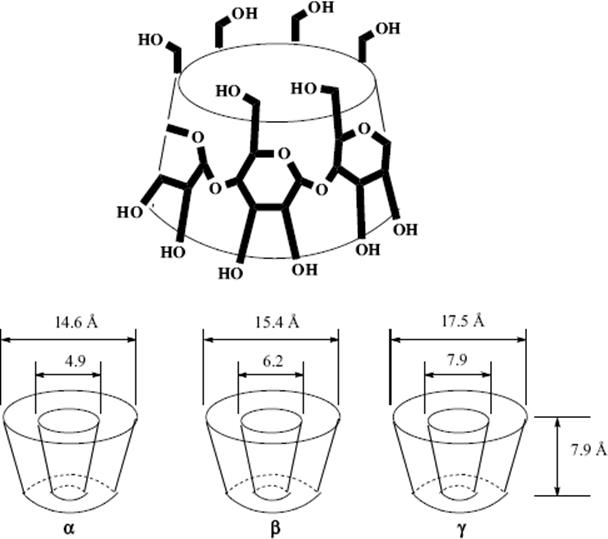
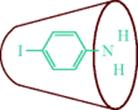
Fig. 3.4 Schematic drawing of the complex of α-cyclodextrin and p-iodoaniline.186
The cyclodextrins also form channel-type complexes, in which the host molecules are stacked on top of each other, like coins in a row.188 For example, α-cyclodextrin (cyclohexaamylose) forms cage complexes with acetic, propionic, and butyric acids, but channel complexes with valeric and higher acids. Capped cyclodextrins are known.189