March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 3. Bonding Weaker Than Covalent
3.D. Catenanes and Rotaxanes190
These compounds contain two or more independent portions that are not bonded to each other by any valence forces, but nevertheless must remain linked. [n]-Catenanes (where n corresponds to the number of
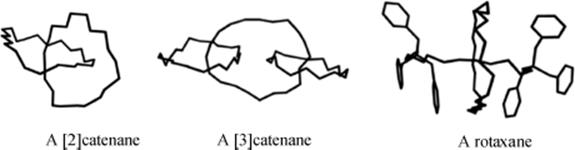
linked rings) are made up of two or more rings held together as links in a chain, while in rotaxanes a linear portion is threaded through a ring and cannot get away because of bulky end groups. Among several types of bulky molecular units, porphyrin units have been used to cap rotaxanes191 as have C60 fullerenes.192 [2]Rotaxanes and [2]catenanes are quite common, and [3]catenanes are known having rather robust amide linkages.193 More intricate variants [e.g., oligocatenanes,194 molecular necklaces (a cyclic oligorotaxane in which a number of small rings are threaded onto a large ring),195 and cyclic daisy chains (an interwoven chain in which each monomer unit acts as a donor and an acceptor for a threading interaction)]196 are known. Ring-in-ring complexes have also been reported.197 Molecular thread, ribbon, and belt assemblies have been synthesized.198 Rotaxanes have been used as the basis for molecular switches,199 and a rotaxane eciplex has been generated that may have applications to molecular-scale photonic devices.200
Transitional isomers are possible in [2]rotaxanes.201 Catenanes and rotaxanes can be prepared by statistical methods or directed syntheses.202 Catenanes can contain heteroatoms and heterocyclic units. In some cases, the catenane exists in equilibrium with the cyclic-non-catenane structures and in some cases this exchange is thought to proceed by ligand exchange and a Möbius strip mechanism.203 An example of a statistical synthesis of a rotaxane is a reaction where a compound A is bonded at two positions to another compound B in the presence of a large ring C. It is hoped that some A molecules would by chance be threaded through C before combining with the two Bmolecules, so that some rotaxane (D) would be formed along with the normal product E.204 In a directed synthesis,205 the separate parts of the molecule are held together by other bonds that are later cleaved.
![]()
Rotation of one unit through the other catenanes is complex, often driven by making and breaking key hydrogen bonds or π–π interactions. In the case of the isophthaloyl [2]catenane (36), the rate-determining steps do not necessarily correspond to the passage of the bulkiest groups.206
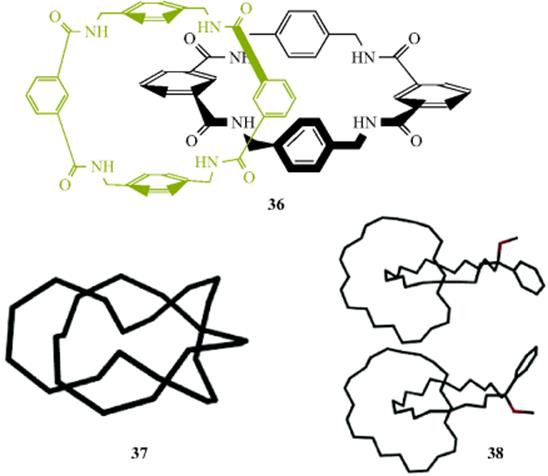
Singly and doubly interlocked [2]catenanes207 can exist as topological stereoisomers208 (see Sec. 4.G for a discussion of diastereomers). Catenanes 37 and 38 are such stereoisomers, and would be expected to have identical mass spectra. Analysis showed that 37 is more constrained and cannot readily accommodate an excess of energy during the mass spectrometry ionization process and, hence, breaks more easily.
Catenanes, molecular knots, and other molecules in these structural categories can exist as enantiomers. In other words, stereoisomers can be generated in some cases. This phenomenon was first predicted by Frisch and Wassermann,209 and the first stereoisomeric catenanes and molecular knots were synthesized by Sauvage and Dietrich-Buchecker.210 Enantiomeric resolution has been achieved.211 A chiral [3]rotaxane containing two achiral wheels, mechanically bonded has been reported,212 generating a cyclodiastereomeric compound, and the enantiomers were separated using chiral HPLC. The terms cycloenantiomerism and cyclodiastereomerism were introduced by Prelog et. al.213 This type of stereoisomerism occurs in cyclic arrangements of several centrally chiral elements in combination with an orientation of the macrocycle.212
A rotaxane can also be an inclusion compound.214 The molecule contains bulky end groups (or “stoppers,” e.g., triisopropylsilyl groups, iPr3Si–) and a chain that consists of a series of –O–CH2CH2–O– groups, but also contains two benzene rings. Cyclodextrins have been threaded onto axle molecules.215 The ring (or bead) around the chain is a macrocycle containing two benzene rings and four pyridine rings. It is preferentially attracted to one of the benzene rings in the chain. The benzene moiety serves as a “station” for the “bead.” However, symmetry of the chain can make the two “stations” equivalent, so that the “bead” is equally attracted to them, and the “bead” actually moves back and forth rapidly between the two “stations,” as shown by the temperature dependence of the NMR spectrum.216 This molecule has been called a molecular shuttle. A copper(I) complexed rotaxane has been prepared with two fullerene (see Sec. 2.L) stoppers.217
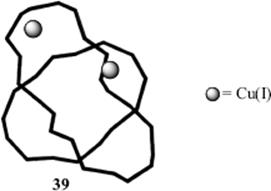
Another variation of these molecules is called molecular knots (e.g., 39), where the represents a metal [in this case, Cu(I)].218 This is particularly interesting since knotted forms of deoxyribonucleic acid (DNA) have been reported.219 There are mechanically interlocked molecules, and one example is known as suit[2]ane.220