March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 4. Stereochemistry and Conformation
4.C. The Fischer Projection
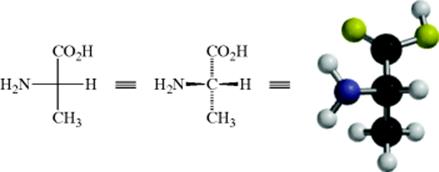
For a thorough understanding of stereochemistry, it is useful to examine molecular models (like those depicted in Fig. 4.1). However, this is not feasible when writing on paper. In 1891, Emil Fischer displayed amino acids and some carbohydrates in a particular way known as a Fischer projection. This is simply a method of representing and “edge-viewed” tetrahedral on paper. By this convention, the model is held so that the two bonds in front of the paper are horizontal and those behind the paper are vertical, as shown for 2-aminopropanoic acid (alanine). With modern computers, molecular models are readily available, but the ability to write structures in two dimensions to represent a 3D form remains important.
In order to obtain proper results with these formulas, remember that they are projections and must be treated differently from the models in testing for superimposability. Every plane is superimposable on its mirror image; hence with these formulas there must be added the restriction that they may not be taken out of the plane of the blackboard or paper. Also, they may not be rotated 90°, although 180° rotation is permissible:
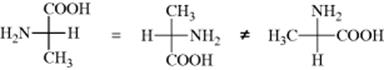
It is also permissible to keep any one group fixed and to rotate the other three clockwise or counterclockwise (because this can be done with models):
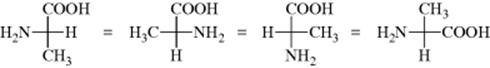
However, the interchange of any two groups results in the conversion of an enantiomer into its mirror image (this applies to models, as well as to the Fischer projections).
With these restrictions Fischer projections may be used instead of models to test whether a molecule containing a stereogenic carbon is superimposable on its mirror image. However, there are no such conventions for molecules whose chirality arises from anything other than chiral atoms (category 5 in Sec. 4.C).