March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 5. Carbocations, Carbanions, Free Radicals, Carbenes, and Nitrenes
5.E. Nitrenes
Nitrenes (R–N),427 are the nitrogen analogues of carbenes, and most of the comments about carbenes also applies to them. Nitrenes are too reactive for isolation under ordinary conditions,428 although ab initio calculations show that nitrenes are more stable than carbenes with an enthalpy difference of 25–26 kcal mol−1 (104.7–108.8 kJ mol−1).429
![]()
Alkyl nitrenes have been isolated by trapping in matrices at 4 K,430 while aryl nitrenes, which are less reactive, can be trapped at 77 K.431 The ground state of NH, and probably of most nitrenes,432 is a triplet, although nitrenes can be generated in both triplet433 and singlet states. A quartet ground-state nitreno radical has been reported.434 In additions of EtOOC–N to C=C double bonds two species are involved, one of which adds in a stereospecific manner and the other not. By analogy with Skell's proposal involving carbenes (Sec. 5.D.i) these are taken to be the singlet and triplet species, respectively.435
The two principal means of generating nitrenes are analogous to those used to form carbenes.
1. Elimination. An example is
![]()
2. Breakdown of Certain Double-Bond Compounds. The most common method of forming nitrenes is photolytic or thermal decomposition of azides,436
![]()
The unsubstituted nitrene (NH) has been generated by photolysis of electric discharge through NH3, N2H4, or HN3.
The reactions of nitrenes are also similar to those of carbenes.437 As in that case, many reactions in which nitrene intermediates are suspected probably do not involve free nitrenes. It is often very difficult to obtain proof in any given case that a free nitrene is or is not an intermediate.
1. Insertion (see Reaction 12-13). Nitrenes, especially acyl nitrenes and sulfonyl nitrenes, can insert into C–H and certain other bonds, for example,

2. Addition to C=C bonds (see Reaction 15-54):
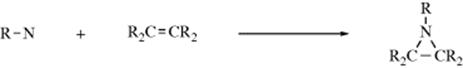
3. Rearrangements.413 Alkyl nitrenes do not generally give either of the two preceding reactions because rearrangement is more rapid, for example,
![]()
Such rearrangements are so rapid that it is usually difficult to exclude the possibility that a free nitrene was never present at all; that is, that migration takes place at the same time the nitrene is formed438 (see Reaction 18-12). However, the rearrangement of naphthylnitrenes to novel bond-shift isomers has been reported.439
4. Abstraction, For example,
![]()
5. Dimerization. One of the principal reactions of NH is dimerization to diimide (N2H2). Azobenzenes are often obtained in reactions where aryl nitrenes are implicated:440
![]()
It would thus seem that dimerization is more important for nitrenes than it is for carbenes, but again it has not been proven that free nitrenes are actually involved.
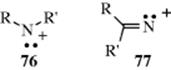
At least two types of nitrenium ions,441 the nitrogen analogues of carbocations, can exist as intermediates, although much less work has been done in this area than on carbocations. In one type (76), the nitrogen is bonded to two atoms (R or R′ can be H),442 and in the other (77) to only one atom.443 When R = H in 76 the species is a protonated nitrene. Like carbenes and nitrenes, nitrenium ions can exist in singlet or triplet states.444
Notes
1. For general references, see Isaacs, N.S. Reactive Intermediates in Organic Chemistry, Wiley, NY, 1974; McManus, S.P. Organic Reactive Intermediates, Academic Press, NY, 1973. Two serial publications devoted to review articles on this subject are Reactive Intermediates (Wiley) and Reactive Intermediates (Plenum).
2. See Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, 5 Vols., Wiley, NY, 1968–1976; Vogel, P. Carbocation Chemistry, Elsevier, NY, 1985. See Saunders, M.; Jiménez-Vázquez, H.A. Chem. Rev. 1991, 91, 375; Arnett, E.M.; Hofelich, T.C.; Schriver, G.W. React. Intermed. (Wiley) 1987, 3, 189. For reviews of dicarbocations, see Lammertsma, K.; Schleyer, P.v.R.; Schwarz, H. Angew. Chem. Int. Ed. 1989, 28, 1321. See also, the series Advances in Carbocation Chemistry.
3. Gomberg, M. Ber. 1902, 35, 2397.
4. For a history of the term “carbonium ion”, see Traynham, J.G. J. Chem. Educ. 1986, 63, 930.
5. Olah, G.A. CHEMTECH 1971, 1, 566; J. Am. Chem. Soc. 1972, 94, 808.
6. Gold, V.; Loening, K.L.; McNaught, A.D.; Sehmi, P. Compendium of Chemical Terminology, IUPAC Recommendations, Blackwell Scientific Publications, Oxford, 1987.
7. Olah, G.A. J. Org. Chem. 2001, 66, 5943. See Olah, G.A.; Prakash, G.K.S. (Eds.), Carbocation Chemistry, Wiley Intersience, Hoboken, NJ, 2004.
8. See Laube, T. J. Am. Chem. 2004, 126, 10904 and references therein. For the X-ray of a vinyl carbocation see Müller, T.; Juhasz, M.; Reed, C.A. Angew. Chem. Int. Ed. 2004, 43, 1543.
9. Kato, T.; Reed, C.A. Angew. Chem. Int. Ed. 2004, 43, 2908.
10. Douberly, G.E.; Ricks, A.M.; Ticknor, B.W.; Schleyer, P.v.R.; Duncan, M.A. J. Am. Chem. Soc. 2007, 129, 13782.
11. Lüning, U.; Baumstark, R. Tetrahedron Lett. 1993, 34, 5059.
12. McClelland, R.A.; Cozens, F.L.; Steenken, S.; Amyes, T.L.; Richard, J.P. J. Chem. Soc. Perkin Trans. 2 1993, 1717.
13. For a treatise, see Szwarc, M. Ions and Ion Pairs in Organic Reactions, 2 Vols., Wiley, NY, 1972–1974.
14. For a review, see Olah, G.A.; Olah, J.A. in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 2, WIley, NY, 1969, pp. 715–782. Also see, Farcasiu, D.; Norton, S.H. J. Org. Chem. 1997, 62, 5374.
15. See Olah, G.A.; Prakash, G.K.S.; Sommer, J. in Superacids, Wiley, NY, 1985, pp. 65–175.
16. Olah, G.A.; Baker, E.B.; Evans, J.C.; Tolgyesi, W.S.; McIntyre, J.S.; Bastien, I.J. J. Am. Chem. Soc. 1964, 86, 1360; Kramer, G.M. J. Am. Chem. Soc. 1969, 91, 4819.
17. Olah, G.A.; Sommer, J.; Namanworth, E. J. Am. Chem. Soc. 1967, 89, 3576.
18. Olah, G.A.; Halpern, Y. J. Org. Chem. 1971, 36, 2354. See also, Herlem, M. Pure Appl. Chem. 1977, 49, 107.
19. Olah, G.A.; Lukas, J. J. Am. Chem. Soc. 1967, 89, 4739.
20. See Amyes, T.L.; Stevens, I.W.; Richard, J.P. J. Org. Chem. 1993, 58, 6057 for a recent study.
21. See Saunders, M.; Hagen, E.L.; Rosenfeld, J. J. Am. Chem. Soc. 1968, 90, 6882; Saunders, M.; Cox, D.; Lloyd, J.R. J. Am. Chem. Soc. 1979, 101, 6656; Myhre, P.C.; Yannoni, C.S. J. Am. Chem. Soc. 1981, 103, 230.
22. Olah, G.A.; Donovan, D.J. J. Am. Chem. Soc. 1978, 100, 5163.
23. Olah, G.A.; Olah, J.A. in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 2, Wiley, NY, 1969, p. 722.
24. Bacon, J.; Gillespie, R.J. J. Am. Chem. Soc. 1971, 91, 6914.
25. See Radom, L.; Poppinger, D.; Haddon, R.C. in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 5, Wiley, NY, 1976, pp. 2303–2426.
26. Lowry, T.H.; Richardson, K.S. Mechanism and Theory in Organic Chemistry, 3rd ed., HarperCollins, NY, 1987, p. 68.
27. Meot-Ner, M. J. Am. Chem. Soc. 1987, 109, 7947.
28. If only the field effect were operating, 2 would be more stable than 3, since deuterium is electron-donating with respect to hydrogen (Sec. 1.J), assuming that the field effect of deuterium could be felt two bonds away.
29. Lambert, J.B.; Ciro, S.M. J. Org. Chem. 1996, 61, 1940.
30. Olah, G.A.; Lukas, J. J. Am. Chem. Soc. 1967, 89, 4739; Olah, G.A.; Olah, J.A. in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 2, Wiley, NY, 1969, pp. 750–764.
31. Olah, G.A.; Klopman, G.; Schlosberg, R.H. J. Am. Chem. Soc. 1969, 91, 3261. See also, Hogeveen, H.; Gaasbeek, C.J. Recl. Trav. Chim. Pays-Bas 1968, 87, 319.
32. Olah, G.A.; Svoboda, J.J.; Ku, A.T. Synthesis 1973, 492; Olah, G.A.; Lukas, J. J. Am. Chem. Soc. 1967, 89, 4739.
33. See Barbour, J.B.; Karty, J.M. J. Org. Chem. 2004, 69, 648; Mo, Y. J. Org. Chem. 2004, 69, 5563 and references cited therein.
34. For reviews, see Deno, N.C. in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 2 Wiley, NY, 1970, pp. 783–806; Richey, Jr., H.G. in Zabicky, J. The Chemistry of Alkenes, Vol. 2, Wiley, NY, 1970, pp. 39–114.
35. Deno, N.C.; Richey, Jr., H.G.; Friedman, N.; Hodge, J.D.; Houser, J.J.; Pittman, Jr., C.U. J. Am. Chem. Soc. 1963, 85, 2991.
36. Olah, G.A.; Spear, R.J. J. Am. Chem. Soc. 1975, 97, 1539 and references cited therein.
37. For a review of divinylmethyl and trivinylmethyl cations, see Sorensen, T.S. in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 2, Wiley, NY, 1970, pp. 807–835.
38. Deno, N.C.; Pittman, Jr., C.U. J. Am. Chem. Soc. 1964, 86, 1871.
39. Olah, G.A.; Spear, R.J.; Westerman, P.W.; Denis, J. J. Am. Chem. Soc. 1974, 96, 5855.
40. For a review of benzylic, diarylmethyl, and triarymethyl cations, see Freedman, H.H. in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 4, Wiley, NY, 1971, pp. 1501–1578.
41. Olah, G.A.; Porter, R.D.; Jeuell, C.L.; White, A.M. J. Am. Chem. Soc. 1972, 94, 2044.
42. Volz, H.; Schnell, H.W. Angew. Chem. Int. Ed. 1965, 4, 873.
43. Deno, N.C.; Schriesheim, A. J. Am. Chem. Soc. 1955, 77, 3051.
44. Prakash, G.K.S. Pure Appl. Chem. 1998, 70, 2001.
45. Ito, S.; Morita, N.; Asao, T. Tetrahedron Lett. 1992, 33, 3773.
46. Ito, S.; Morita, N.; Asao, T. Tetrahedron Lett. 1994, 35, 751.
47. For reviews, see in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 3, Wiley, NY, 1972: Richey, Jr., H.G. pp. 1201–294; Wiberg, K.B.; Hess, Jr., B.A.; Ashe, III, A.H. pp. 1295–1345.
48. Pittman, Jr., C.U.; Olah, G.A. J. Am. Chem. Soc. 1965, 87, 2998; Deno, N.C.; Liu, J.S.; Turner, J.O.; Lincoln, D.N.; Fruit, Jr., R.E. J. Am. Chem. Soc. 1965, 87, 3000.
49. Deno, N.C.; Richey, Jr., H.G.; Liu, J.S.; Hodge, J.D.; Houser, H.J.; Wisotsky, M.J. J. Am. Chem. Soc. 1962, 84, 2016.
50. See Poulter, C.D.; Spillner, C.J. J. Am. Chem. Soc. 1974, 96, 7591; Childs, R.F.; Kostyk, M.D.; Lock, C.J.L.; Mahendran, M. J. Am. Chem. Soc. 1990, 112, 8912.
51. Sorensen, T.S.; Miller, I.J.; Ranganayakulu, K. Aust. J. Chem. 1973, 26, 311.
52. See Hevesi, L. Bull. Soc. Chim. Fr. 1990, 697; Olah, G.A.; Liang, G.; Mo, Y.M. J. Org. Chem. 1974, 39, 2394; Borch, R.F. J. Am. Chem. Soc. 1968, 90, 5303; Rabinovitz, M.; Bruck, D. Tetrahedron Lett. 1971, 245.
53. For a review of ions of the form R2C+–OR', see Rakhmankulov, D.L.; Akhmatdinov, R.T.; Kantor, E.A. Russ. Chem. Rev. 1984, 53, 888. For a review of ions of the form R'C+(OR)2 and C+(OR)3, see Pindur, U.; Müller, J.; Flo, C.; Witzel, H. Chem. Soc. Rev. 1987, 16, 75.
54. For a review of such ions where nitrogen is the heteroatom, see Scott, F.L.; Butler, R.N. in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 4, Wiley, NY, 1974, pp. 1643–1696.
55. See Allen, A.D.; Tidwell, T.T. Adv. Carbocation Chem. 1989, 1, 1. See also, Teberekidis, V.I.; Sigalas, M.P. Tetrahedron 2003, 59, 4749.
56. Olah, G.A.; Svoboda, J.J. Synthesis 1973, 52.
57. See Lambert, J.B. Tetrahedron 1990, 46, 2677; Lambert, J.B.; Zhao, Y.; Emblidge, R.W.; Salvador, L.A.,;Liu, X.; So, J.-H.; Chelius, E.C. Acc. Chem. Res.1999, 32, 183. See also, Lambert, J.B.; Chelius, E.C. J. Am. Chem. Soc.1990, 112, 8120.
58. Creary, X.; Kochly, E.D. J. Org. Chem. 2009, 74, 9044.
59. Olah, G.A.; Heiliger, L.; Prakash, G.K.S. J. Am. Chem. Soc. 1989, 111, 8020.
60. Haubenstock, H.; Sauers, R.R. Tetrahedron 2004, 60, 1191.
61. see Al-Talib, M.; Tashtoush, H. Org. Prep. Proced. Int. 1990, 22, 1; Olah, G.A.; Germain, A.; White, A.M. in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 5, Wiley, NY, 1976, pp. 2049–2133; Lindner, E. Angew. Chem. Int. Ed. 1970, 9, 114.
62. See Olah, G.A.; Dunne, K.; Mo, Y.K.; Szilagyi, P. J. Am. Chem. Soc. 1972, 94, 4200; Olah, G.A.; Svoboda, J.J. Synthesis 1972, 306.
63. Hammett, L.P.; Deyrup, A.J. J. Am. Chem. Soc. 1933, 55, 1900; Newman, M.S.; Deno, N.C. J. Am. Chem. Soc. 1951, 73, 3651.
64. Boer, F.P. J. Am. Chem. Soc. 1968, 90, 6706; Le Carpentier, J.; Weiss, R. Acta Crystallogr. Sect. B, 1972, 1430. See also, Olah, G.A.; Westerman, P.W. J. Am. Chem. Soc. 1973, 95, 3706.
65. See Komatsu, K.; Kitagawa, T. Chem. Rev. 2003, 103, 1371. Also see, Gilbertson, R.D.; Weakley, T.J.R.; Haley, M.M. J. Org. Chem. 2000, 65, 1422.
66. See Gronheid, R.; Lodder, G.; Okuyama, T. J. Org. Chem. 2002, 67, 693. For a discussion of aryl substituted vinyl cations, see Müller, T.; Margraf, D.; Syha, Y. J. Am. Chem. Soc. 2005, 127, 10852.
67. For a review of destabilized carbocations, see Tidwell, T.T. Angew. Chem. Int. Ed. 1984, 23, 20.
68. See Abram, T.S.; Watts, W.E. J. Chem. Soc. Chem. Commun,. 1974, 857; Siehl, H.; Carnahan, Jr., J.C.; Eckes, L.; Hanack, M. Angew. Chem. Int. Ed. 1974, 13, 675. Also see Franke, W.; Schwarz, H.; Stahl, D. J. Org. Chem.1980, 45, 3493. See also, Siehl, H.; Koch, E. J. Org. Chem. 1984, 49, 575.
69. See Stang, P.J.; Rappoport, Z.; Hanack, M.; Subramanian, L.R. Vinyl Cations, Academic Press, NY, 1979; Hanack, M. Pure Appl. Chem. 1984, 56, 1819, Acc. Chem. Res. 1976, 9, 364; Ambroz, H.B.; Kemp, T.J. Chem. Soc. Rev. 1979, 8, 353; Richey, Jr., H.G.; Richey, J.M. in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 2, Wiley, NY, 1970, pp. 899–957; Richey, Jr., H.G. in Zabicky, J. The Chemistry of Alkenes, Vol. 2, Wiley, NY, 1970, pp. 42–49; Stang, P.J. Prog. Phys. Org. Chem. 1973, 10, 205. See also, Charton, M. Mol. Struct. Energ. 1987, 4, 271. For a computational study, see Glaser, R.; Horan, C. J.; Lewis, M.; Zollinger, H. J. Org. Chem. 1999, 64, 902.
70. Yang, S.; Kondo, J.N.; Domen, K. Chem. Commun. 2001, 2008.
71. Winkler, M.; Sander, W. J. Org. Chem. 2006, 71, 6357.
72. For reviews, see Bagno, A.; Scorrano, G.; More O'Ferrall, R.A. Rev. Chem. Intermed. 1987, 7, 313; Bethell, D.; Gold, V. Carbonium Ionds, Academic Press, NY, 1967, pp. 59–87.
73. Deno, N.C.; Berkheimer, H.E.; Evans, W.L.; Peterson, H.J. J. Am. Chem. Soc. 1959, 81, 2344.
74. For a list of stabilities of 39 typical carbocations, see Arnett, E.M.; Hofelich, T.C. J. Am. Chem. Soc. 1983, 105, 2889. See also, Schade, C.; Mayr, H.; Arnett, E.M. J. Am. Chem. Soc. 1988, 110, 567; Schade, C.; Mayr, H. Tetrahedron 1988, 44, 5761.
75. Hammett, L.P.; Deyrup, A.J. J. Am. Chem. Soc. 1933, 55, 1900; Newman, M.S.; Deno, N.C. J. Am. Chem. Soc. 1951, 73, 3651; Boer, F.P. J. Am. Chem. Soc. 1968, 90, 6706; Le Carpentier, J.; Weiss, R. Acta Crystallogr. Sect. B, 1972, 1430. See also, Arnett, E.M.; Petro, C. J. Am. Chem. Soc. 1978, 100, 5408; Arnett, E.M.; Pienta, N.J. J. Am. Chem. Soc. 1980, 102, 3329.
76. Schultz, J.C.; Houle, F.A.; Beauchamp, J.L. J. Am. Chem. Soc. 1984, 106, 3917.
77. Lossing. F.P.; Holmes, J.L. J. Am. Chem. Soc. 1984, 106, 6917.
78. Vinyl cations are generated by photolysis of vinyl iodonium salts. See Slegt, M.; Gronheid, R.; van der Vlugt, D.; Ochiai, M.; Okuyama, T.; Zuilhof, H.; Overkleeft, H.S.; Lodder, G. J. Org. Chem. 2006, 71, 2227.
79. See Schleyer, P.v.R. in Chiurdoglu, G. Conformational Analysis, Academic Press, NY, 1971, p. 241; Hehre, W.J. Acc. Chem. Res. 1975, 8, 369; Freedman, H.H. in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 4, Wiley, NY, 1974, pp. 1561–574.
80. Olah, G.A.; DeMember, J.R.; Commeyras, A.; Bribes, J.L. J. Am. Chem. Soc. 1971, 93, 459; Yannoni, C.S.; Kendrick, R.D.; Myhre, P.C.; Bebout, D.C.; Petersen, B.L. J. Am. Chem. Soc. 1989, 111, 6440.
81. Rauk, A.; Sorensen, T.S.; Maerker, C.; de M. Carneiro, J.W.; Sieber, S.; Schleyer, P.v.R. J. Am. Chem. Soc. 1996, 118, 3761.
82. Lawlor, D.A.; More O'Ferrall, R.A.; Rao, S.N. J. Am. Chem. Soc. 2008, 130, 17997.
83. For a review of bridgehead carbocations, see Fort, Jr., R.C. in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 4, Wiley, NY, 1974, pp. 1783–1835.
84. Della, E.W.; Schiesser, C.H. J. Chem. Soc. Chem. Commun. 1994, 417.
85. Åhman, J.; Somfai, P.; Tanner, D. J. Chem. Soc. Chem. Commun. 1994, 2785.
86. Della, E.W.; Head, N.J.; Janowski, W.K.; Schiesser, C.H. J. Org. Chem. 1993, 58, 7876.
87. Olah, G.A.; Prakash, G.K.S.; Shih, J.G.; Krishnamurthy, V.V.; Mateescu, G.D.; Liang, G.; Sipos, G.; Buss, V.; Gund, T.M.; Schleyer, P.v.R. J. Am. Chem. Soc. 1985, 107, 2764. See also, Kruppa, G.H.; Beauchamp, J.L. J. Am. Chem. Soc. 1986, 108, 2162; Laube, T. Angew. Chem. Int. Ed. 1986, 25, 349.
88. Takeuchi, K.; Okazaki, T.; Kitagawa, T.; Ushino, T.; Ueda, K.; Endo, T.; Notario, R. J. Org. Chem. 2001, 66, 2034.
89. Olah, G.A.; Prakash, G.K.S.; Fessner, W.; Kobayashi, T.; Paquette, L.A. J. Am. Chem. Soc. 1988, 110, 8599.
90. de Meijere, A.; Schallner, O. Angew. Chem. Int. Ed. 1973, 12, 399.
91. See Sundaralingam, M.; Chwang, A.K. in Olah, G.A.; Schleyer, P.v.R. Carbonium Ions, Vol. 5, Wiley, NY, 1976, pp. 2427–2476.
92. Schuster, I.I.; Colter, A.K.; Kurland, R.J. J. Am. Chem. Soc. 1968, 90, 4679.
93. For reviews of the NMR spectra of carbocations, see Young, R.N. Prog. Nucl. Magn. Reson. Spectrosc., 1979, 12, 261; Farnum, D.G. Adv. Phys. Org. Chem. 1975, 11, 123.
94. Olah, G.A.; White, A.M. J. Am. Chem. Soc. 1968, 90, 1884; 1969, 91, 5801. For 13C NMR data for additional ions, see Olah, G.A.; Donovan, D.J. J. Am. Chem. Soc. 1977, 99, 5026; Olah, G.A.; Prakash, G.K.S.; Liang, G. J. Org. Chem. 1977, 42, 2666.
95. Olah, G.A.; Porter, R.D.; Kelly, D.P. J. Am. Chem. Soc. 1971, 93, 464.
96. See Brown, H.C.; Peters, E.N. J. Am. Chem. Soc. 1977, 99, 1712; Kitching, W.; Adcock, W.; Aldous, G. J. Org. Chem. 1979, 44, 2652. See also, Larsen, J.W.; Bouis, P.A. J. Am. Chem. Soc. 1975, 97, 4418; Volz, H.; Shin, J.; Streicher, H. Tetrahedron Lett. 1975, 1297; Larsen, J.W. J. Am. Chem. Soc. 1978, 100, 330.
97. Peterson, P.E.; Slama, F.J. J. Am. Chem. Soc., 1968, 90, 6516.
98. Carlier, P.R.; Deora, N.; Crawford, T.D. J. Org. Chem. 2006, 71, 1592.
99. Mascal, M.; Hafezi, N.; Meher N.K.; Fettinger, J.C. J. Am. Chem. Soc. 2008, 130, 13532.
100. Prakash, G.K.S.; Bae, C.; Rasul, G.; Olah, G.A. J. Org. Chem. 2002, 67, 1297.
101. Richard, J.P.; Amyes, T.L.; Williams, K.B. Pure. Appl. Chem. 1998, 70, 2007.
102. Toteva, M.M.; Richard, J.P. J. Am. Chem. Soc. 1996, 118, 11434.
103. Vrcek, V.; Saunders, M.; Kronja, O. J. Am. Chem. Soc. 2004, 126, 13703.
104. Kronja, O.; Kohli, T.-P.; Mayr, H.; Saunders, M. J. Am. Chem. Soc. 2000, 122, 8067.
105. See Buncel, E.; Durst, T. Comprehensive Carbanion Chemistry, pts. A, B, and C; Elsevier, NY, 1980, 1984, 1987; Bates, R.B.; Ogle, C.A. Carbanion Chemistry, Springer, NY, 1983; Stowell, J.C. Carbanions in Organic Synthesis, Wiley, NY, 1979; Cram, D.J. Fundamentals of Carbanion Chemistry, Academic Press, NY, 1965; Staley, S.W. React. Intermed. (Wiley) 1985, 3, 19; Staley, S.W.; Dustman, C.K. React. Intermed. (Wiley) 1981, 2, 15. For reviews of NMR spectra of carbanions, see Young, R.N. Prog. Nucl. Magn. Reson. Spectrosc. 1979, 12, 261. For a review of dicarbanions, see Thompson, C.M.; Green, D.L.C. Tetrahedron 1991, 47, 4223.
106. See Reutov, O.A.; Beletskaya, I.P.; Butin, K.P. CH-Acids, Pergamon, Elmsford, NY, 1978; Fischer, H.; Rewicki, D. Prog. Org. Chem. 1968, 7, 116.
107. See Graul, S.T.; Squires, R.R. J. Am. Chem. Soc. 1988, 110, 607.
108. Applequist, D.E.; O'Brien, D.F. J. Am. Chem. Soc. 1963, 85, 743.
109. Dessy, R.E.; Kitching, W.; Psarras, T.; Salinger, R.; Chen, A.; Chivers, T. J. Am. Chem. Soc. 1966, 88, 460.
110. Terrier, F.; Magnier, E.; Kizilian, E.; Wakselman, C.; Buncel, E. J. Am. Chem. Soc. 2005, 127, 5563.
111. For reviews, see Jones, J.R. Surv. Prog. Chem. 1973, 6, 83; Shatenshtein, A.I.; Shapiro, I.O. Russ. Chem. Rev. 1968, 37, 845.
112. See Bordwell, F.G.; Matthews, W.S.; Vanier, N.R. J. Am. Chem. Soc. 1975, 97, 442.
113. DePuy, C.H.; Gronert, S.; Barlow, S.E.; Bierbaum, V.M.; Damrauer, R. J. Am. Chem. Soc. 1989, 111, 1968. The same order (for t-Bu, Me, iPr, and Et) was found in gas-phase cleavages of alkoxides (Reaction 12-41): Tumas, W.; Foster, R.F.; Brauman, J.I. J. Am. Chem. Soc. 1984, 106, 4053.
114. Graul, S.T.; Squires, R.R. J. Am. Chem. Soc. 1988, 110, 607.
115. See Richey, Jr., H.G. in Zabicky, J. The Chemistry of Alkenes, Vol. 2, Wiley, NY, 1970, pp. 67–77.
116. See Bockrath, B.; Dorfman, L.M. J. Am. Chem. Soc. 1974, 96, 5708.
117. See Buncel, E.; Menon, B. in Buncel, E.; Durst, T. Comprehensive Carbanion Chemistry, pts. A, B, and C, Elsevier, NY, 1980, 1984, 1987, pp. 97–124.
118. Olmstead, M.M.; Power, P.P. J. Am. Chem. Soc. 1985, 107, 2174.
119. Laferriere, M.; Sanrame, C. N.; Scaiano, J. C. Org. Lett. 2004, 6, 873.
120. Kinoshita, T.; Fujita, M.; Kaneko, H.; Takeuchi, K-i.; Yoshizawa, K.; Yamabe, T. Bull. Chem. Soc. Jpn. 1998, 71, 1145.
121. Eldin, S.; Whalen, D.L.; Pollack, R.M. J. Org. Chem. 1993, 58, 3490.
122. Abbotto, A.; Bradamante, S.; Pagani, G.A. J. Org. Chem. 1993, 58, 449.
123. Perkins, M.J.; Peynircioglu, N.B. Tetrahedron 1985, 41, 225.
124. Okamoto, K.; Kitagawa, T.; Takeuchi, K.; Komatsu, K.; Kinoshita, T.; Aonuma, S.; Nagai, M.; Miyabo, A. J. Org. Chem. 1990, 55, 996. See also, Okamoto, K.; Kitagawa, T.; Takeuchi, K.; Komatsu, K.; Miyabo, A. J. Chem. Soc. Chem. Commun. 1988, 923.
125. See Richey, Jr., H.G. in Zabicky, J. The Chemistry of Alkenes, Vol. 2, Wiley, NY, 1970, pp. 49–56.
126. See Oae, S.; Uchida, Y. in Patai, S.; Rappoport, Z.; Stirling, C. The Chemistry of Sulphones and Sulphoxides, Wiley, NY, 1988, pp. 583–664; Wolfe, S. in Bernardi, F.; Csizmadia, I.G.; Mangini. A. Organic Sulfur Chemistry, Elsevier, NY, 1985, pp. 133–190; Block, E. Reactions of Organosulfur Compounds, Academic Press, NY, 1978, pp. 42–56; Durst, T.; Viau, R. Intra-Sci. Chem. Rep. 1973, 7 (3), 63. Also see, Reich, H.J. in Liotta, DC. Organoselenium Chemistry, Wiley, NY, 1987, pp. 243–276.
127. See Wolfe, S.; LaJohn, L.A.; Bernardi, F.; Mangini, A.; Tonachini, G. Tetrahedron Lett. 1983, 24, 3789; Wolfe, S.; Stolow, A.; LaJohn, L.A. Tetrahedron Lett. 1983, 24, 4071.
128. See Borden, W.T.; Davidson, E.R.; Andersen, N.H.; Denniston, A.D.; Epiotis, N.D. J. Am. Chem. Soc. 1978, 100, 1604; Bernardi, F.; Bottoni, A.; Venturini, A.; Mangini, A. J. Am. Chem. Soc. 1986, 108, 8171.
129. Bernasconi, C.F.; Kittredge, K.W. J. Org. Chem. 1998, 63, 1944.
130. Wetzel, D.M.; Brauman, J.I. J. Am. Chem. Soc. 1988, 110, 8333.
131. For a review of such carbanions, see Beak, P.; Reitz, D.B. Chem. Rev. 1978, 78, 275. See also, Rondan, N.G.; Houk, K.N.; Beak, P.; Zajdel, W.J.; Chandrasekhar, J.; Schleyer, P.v.R. J. Org. Chem. 1981, 46, 4108.
132. See Werstiuk, N.H. Tetrahedron 1983, 39, 205; Hunter, D.H.; Stothers, J.B.; Warnhoff, E.W. in de Mayo, P. Rearrangements in Ground and Excited States, Vol. 1, Academic Press, NY, 1980, pp. 410–437.
133. See Werstiuk, N.H.; Yeroushalmi, S.; Timmins, G. Can. J. Chem. 1983, 61, 1945; Lee, R.E.; Squires, R.R. J. Am. Chem. Soc. 1986, 108, 5078; Peiris, S.; Ragauskas, A.J.; Stothers, J.B. Can. J. Chem. 1987, 65, 789; Shiner, C.S.; Berks, A.H.; Fisher, A.M. J. Am. Chem. Soc. 1988, 110, 957.
134. For reviews of carbanion pairs, see Hogen-Esch, T.E. Adv. Phys. Org. Chem. 1977, 15, 153; Jackman, L.M.; Lange, B.C. Tetrahedron 1977, 33, 2737. See also, Laube, T. Acc. Chem. Res. 1995, 28, 399.
135. Zook, H.D.; Gumby, W.L. J. Am. Chem. Soc. 1960, 82, 1386.
136. Solov'yanov, A.A.; Karpyuk, A.D.; Beletskaya, I.P.; Reutov, O.A. J. Org. Chem. USSR 1981, 17, 381. See also, Solov'yanov, A.A.; Beletskaya, I.P.; Reutov, O.A. J. Org. Chem. USSR 1983, 19, 1964.
137. See DePalma, V.M.; Arnett, E.M. J. Am. Chem. Soc. 1978, 100, 3514; Buncel, E.; Menon, B. J. Org. Chem. 1979, 44, 317; O'Brien, D.H.; Russell, C.R.; Hart, A.J. J. Am. Chem. Soc. 1979, 101, 633; Streitwieser, Jr., A.; Shen, C.C.C. Tetrahedron Lett. 1979, 327; Streitwieser, Jr., A. Acc. Chem. Res. 1984, 17, 353.
138. See Schade, C.; Schleyer, P.v.R.; Geissler, M.; Weiss, E. Angew. Chem. Int. Ed. 1986, 21, 902.
139. Ellison, G.B.; Engelking, P.C.; Lineberger, W.C. J. Am. Chem. Soc. 1978, 100, 2556.
140. Retention of configuration has never been observed with simple carbanions. Cram has obtained retention with carbanions stabilized by resonance. However, these carbanions are known to be planar or nearly planar, and retention was caused by asymmetric solvation of the planar carbanions (see Sec. 12.A.ii).
141. See Peoples, P.R.; Grutzner, J.B. J. Am. Chem. Soc. 1980, 102, 4709.
142. See Feit, B.; Melamed, U.; Speer, H.; Schmidt, R.R. J. Chem. Soc. Perkin Trans. 1 1984, 775; Chou, P.K.; Kass, S.R. J. Am. Chem. Soc. 1991, 113, 4357.
143. Boche, G.; Harms, K.; Marsch, M. J. Am. Chem. Soc. 1988, 110, 6925; Boche, G.; Walborsky, H.M. Cyclopropane Derived Reactive Intermediates, Wiley, NY, 1990. For a review, see Boche, G.; Walborsky, H.M. in Rappoport, Z. The Chemistry of the Cyclopropyl Group, pt. 1, Wiley, NY, 1987, pp. 701–808.
144. See Cram, D.J. Fundamentals of Carbanion Chemistry, Academic Press, NY, 1965, pp. 85–105.
145. Bordwell, F.G.; Phillips, D.D.; Williams, Jr., J.M. J. Am. Chem. Soc. 1968, 90, 426; Annunziata, R.; Cinquini, M.; Colonna, S.; Cozzi, F. J. Chem. Soc. Chem. Commun. 1981, 1005; Chassaing, G.; Marquet, A.; Corset, J.; Froment, F. J. Organomet. Chem. 1982, 232, 293; Cram, D.J. Fundamentals of Carbanion Chemistry, Academic Press, NY, 1965, pp. 105–113; Hirsch, R.; Hoffmann, R.W. Chem. Ber. 1992, 125, 975.
146. Hoffmann, R.W.; Rühl, T.; Chemla, F.; Zahneisen, T. Liebigs Ann. Chem. 1992, 719.
147. Rychnovsky, S.D.; Plzak, K.; Pickering, D. Tetrahedron Lett. 1994, 35, 6799.
148. Reich, H.J.; Medina, M.A.; Bowe, M.D. J. Am. Chem. Soc. 1992, 114, 11003.
149. Jenkins, P.R.; Symons, M.C.R.; Booth, S.E.; Swain, C.J. Tetrahedron Lett. 1992, 33, 3543.
150. Gais, H.; Müller, J.; Vollhardt, J.; Lindner, H.J. J. Am. Chem. Soc. 1991, 113, 4002. For a contrary view, see Trost, B.M.; Schmuff, N.R. J. Am. Chem. Soc. 1985, 107, 396.
151. Grossert, J.S.; Hoyle, J.; Cameron, T.S.; Roe, S.P.; Vincent, B.R. Can. J. Chem. 1987, 65, 1407.
152. See Elschenbroich, C.; Salzer, A. Organometallics, VCH, NY, 1989; Oliver, J.P. in Hartley, F.R.; Patai, S. The Chemistry of the Metal–Carbon Bond, Vol. 2, Wiley, NY, 1985, pp. 789–826; Coates, G.E.; Green, M.L.H.; Wade, K. Organometallic Compounds, 3rd ed., Vol. 1, Methuen: London, 1967; Grovenstein, Jr., E. in Buncel, E.; Durst, T. Comprehensive Carbanion Chemistry, pt. C, Elsevier, NY, 1987, pp. 175–221.
153. See Schade, C.; Schleyer, P.v.R. Adv. Organomet. Chem. 1987, 27, 169.
154. For X-ray crystallography studies, see Weiss, E.; Sauermann, G. Chem. Ber. 1970, 103, 265; Weiss, E.; Köster, H. Chem. Ber. 1977, 110, 717.
155. See Setzer, W.N.; Schleyer, P.v.R. Adv. Organomet. Chem. 1985, 24, 353; Schleyer, P.v.R. Pure Appl. Chem. 1984, 56, 151; Brown, T.L. Pure Appl. Chem. 1970, 23, 447, Adv. Organomet. Chem. 1965, 3, 365; Kovrizhnykh, E.A.; Shatenshtein, A.I. Russ. Chem. Rev. 1969, 38, 840. For reviews of the structures of lithium enolate anions and related compounds, see Boche, G. Angew. Chem. Int. Ed. 1989, 28, 277; Seebach, D. Angew. Chem. Int. Ed. 1988, 27, 1624. Also see Günther, H.; Moskau, D.; Bast, P.; Schmalz, D. Angew. Chem. Int. Ed. 1987, 26, 1212; Wakefield, B.J. Organolithium Methods, Academic Press, NY, 1988, The Chemistry of Organolithium Compounds, Pergamon, Elmsford, NY, 1974.
156. Lewis, H.L.; Brown, T.L. J. Am. Chem. Soc. 1970, 92, 4664; Brown, T.L.; Rogers, M.T. J. Am. Chem. Soc. 1957, 79, 1859; Weiner, M.A.; Vogel, G.; West, R. Inorg. Chem. 1962, 1, 654.
157. Thomas, R.D.; Jensen, R.M.; Young, T.C. Organometallics 1987, 6, 565. See also, Kaufman, M.J.; Gronert, S.; Streitwieser, Jr., A. J. Am. Chem. Soc. 1988, 110, 2829.
158. Wittig, G.; Meyer, F.J.; Lange, G. Liebigs Ann. Chem. 1951, 571, 167. See also, Bates, T.F.; Clarke, M.T.; Thomas, R.D. J. Am. Chem. Soc. 1988, 110, 5109.
159. Plavš i![]() , D.; Srzi
, D.; Srzi![]() , D.; Klasinc, L. J. Phys. Chem. 1986, 90, 2075.
, D.; Klasinc, L. J. Phys. Chem. 1986, 90, 2075.
160. Weiss, E.; Sauermann, G.; Thirase, G. Chem. Ber. 1983, 116, 74.
161. Bauer, W.; Winchester, W.R.; Schleyer, P.v.R. Organometallics 1987, 6, 2371.
162. Fraenkel, G.; Chow, A.; Winchester, W.R. J. Am. Chem. Soc. 1990, 112, 6190.
163. For reviews, see Ashby, E.C. Bull. Soc. Chim. Fr. 1972, 2133; Q. Rev. Chem. Soc. 1967, 21, 259; Wakefield, B.J. Organomet. Chem. Rev. 1966, 1, 131; Bell, N.A. Educ. Chem. 1973, 143.
164. Schlenk, W.; Schlenk, Jr., W. Ber. 1929, 62B, 920.
165. See Parris, G.; Ashby, E.C. J. Am. Chem. Soc. 1971, 93, 1206; Salinger, R.M.; Mosher, H.S. J. Am. Chem. Soc. 1964, 86, 1782.
166. Guggenberger, L.J.; Rundle, R.E. J. Am. Chem. Soc. 1968, 90, 5375.
167. See Sakamoto, S.; Imamoto, T.; Yamaguchi, K. Org. Lett. 2001, 3, 1793.
168. Weiss, E. Chem. Ber. 1965, 98, 2805.
169. Ashby, E.C.; Smith, M.B. J. Am. Chem. Soc. 1964, 86, 4363; Vreugdenhil, A.D.; Blomberg, C. Recl. Trav. Chim. Pays-Bas 1963, 82, 453, 461.
170. Benn, R.; Lehmkuhl, H.; Mehler, K.; Rufi![]() ska, A. Angew. Chem. Int. Ed. 1984, 23, 534.
ska, A. Angew. Chem. Int. Ed. 1984, 23, 534.
171. Smith, M.B.; Becker, W.E. Tetrahedron 1966, 22, 3027.
172. Evans, D.F.; Fazakerley, V. Chem. Commun. 1968, 974.
173. Ducom, J. Bull. Chem. Soc. Fr. 1971, 3518, 3523, 3529.
174. See Parris, G.; Ashby, E.C. J. Am. Chem. Soc. 1971, 93, 1206.
175. Ashby, E.C.; Walker, F. J. Org. Chem. 1968, 33, 3821.
176. Parris, G.; Ashby, E.C. J. Am. Chem. Soc. 1971, 93, 1206.
177. Ashby, E.C.; Smith, M.B. J. Am. Chem. Soc. 1964, 86, 4363.
178. Fraenkel, G.; Cottrell, C.E.; Dix, D.T. J. Am. Chem. Soc. 1971, 93, 1704; Pechhold, E.; Adams, D.G.; Fraenkel, G. J. Org. Chem. 1971, 36, 1368; Maercker, A.; Geuss, R. Angew. Chem. Int. Ed. 1971, 10, 270.
179. See Pratt, L.M.; Kass, S.R. J. Org. Chem. 2004, 69, 2123.
180. Nichols, M.A.; Williard, P.G. J. Am. Chem. Soc. 1993, 115, 1568.
181. See Jones, A.C.; Sanders, A.W.; Bevan, M.J.; Reich, H.J. J. Am. Chem. Soc. 2007, 129, 3492.
182. Siemeling, U.; Redecker, T.; Neumann, B.; Stammler, H.-G. J. Am. Chem. Soc. 1994, 116, 5507.
183. Ruhlandt-Senge, K.; Ellison, J.J.; Wehmschulte, R.J.; Pauer, F.; Power, P.P. J. Am. Chem. Soc. 1993, 115, 11353. Also see Betz, J.; Hampel, F.; Bauer, W. Org. Lett. 2000, 2, 3805.
184. Sorger, K.; Bauer, W.; Schleyer, P.v.R.; Stalke, D. Angew. Chem. Int. Ed., 1995, 34, 1594.
185. Reich, H.J.; Gudmundsson, B.O.; Goldenberg, W.S.; Sanders, A.W.; Kulicke, K.J.; Simon, K.; Guzei, I. A. J. Am. Chem. Soc. 2001, 123, 8067.
186. Nájera, C.; Yus, M. Tetrahedron 2005, 61, 3137.
187. See Parisel, O.; Fressigne, C.; Maddaluno, J.; Giessner-Prettre, C. J. Org. Chem. 2003, 68, 1290.
188. Sekiguchi, A.; Tanaka, M. J. Am. Chem. Soc. 2003, 125, 12684.
189. Linti, G.; Rodig, A.; Pritzkow, H. Angew. Chem. Int. Ed. 2002, 41, 4503.
190. Reich, H.J.; Green, D.P.; Medina, M.A.; Goldenberg, W.S.; Gudmundsson, B.Ö.; Dykstra, R.R.; Phillips. N.H. J. Am. Chem. Soc. 1998, 120, 7201.
191. Sun, X.; Winemiller, M.D.; Xiang, B.; Collum, D.B. J. Am. Chem. Soc. 2001, 123, 8039. See also, Rutherford, J.L.; Hoffmann, D.; Collum, D.B. J. Am. Chem. Soc. 2002, 124, 264.
192. Piffl, M.; Weston, J.; Günther, W.; Anders, E. J. Org. Chem. 2000, 65, 5942.
193. Bauer, W.; Griesinger, C. J. Am. Chem. Soc. 1993, 115, 10871.
194. Fraenkel, G.; Chow, A.; Fleischer, R.; Liu, H. J. Am. Chem. Soc. 2004, 126, 3983.
195. Graña, P.; Paleo, M.R.; Sardina, F.J. J. Am. Chem. Soc. 2002, 124, 12511.
196. Basu, A.; Thayumanavan, S. Angew. Chem. Int. Ed. 2002, 41, 717. See also, Fraenkel, G.; Duncan, J.H.; Martin, K.; Wang, J. J. Am. Chem. Soc. 1999, 121, 10538.
197. Stork, G.; Hudrlik, P.F. J. Am. Chem. Soc. 1968, 90, 4464; Bernstein, M.P.; Collum, D.B. J. Am. Chem. Soc. 1993, 115, 789; Collum, D.B. Acc. Chem. Res. 1992, 25, 448.
198. Jackman, L.M.; Lange, B.C. J. Am. Chem. Soc. 1981, 103, 4494.
199. Jackman, L.M.; Lange, B.C. Tetrahedron 1977, 33, 2737.
200. Williard, P.G.; Carpenter, G.B. J. Am. Chem. Soc. 1986, 108, 462; Williard, P.G.; Carpenter, G.B. J. Am. Chem. Soc. 1985, 107, 3345 and references cited therein.
201. Seebach, D.; Amstutz, R.; Laube, T.; Schweizer, W.B.; Dunitz, J.D. J. Am. Chem. Soc. 1985, 107, 5403.
202. Abu-Hasanayn, F.; Streitwieser, A. J. Am. Chem. Soc. 1996, 118, 8136.
203. Abbotto, A.; Streitwieser, A.; Schleyer, P.v.R. J. Am. Chem. Soc. 1997, 119, 11255.
204. Carlier, P.R.; Lucht, B.L.; Collum, D.B. J. Am. Chem. Soc. 1994, 116, 11602.
205. DeLong, G.T.; Pannell, D.K.; Clarke, M.T.; Thomas, R.D. J. Am. Chem. Soc. 1993, 115, 7013.
206. Walborsky, H.M.; Hamdouchi, C. J. Org. Chem. 1993, 58, 1187.
207. For a review of such reactions, see Durst, T. in Buncel, E.; Durst, T. Comprehensive Carbanion Chemistry, pt. B, Elsevier, NY, 1984, pp. 239–291.
208. For a review, see Guthrie, R.D. in Buncel, E.; Durst, T. Comprehensive Carbanion Chemistry, pt. A, Elsevier, NY, 1980, pp. 197–269.
209. Arnett, E.M.; Molter, K.E.; Marchot, E.C.; Donovan, W.H.; Smith, P. J. Am. Chem. Soc. 1987, 109, 3788.
210. Okamoto, K.; Kitagawa, T.; Takeuchi, K.; Komatsu, K.; Kinoshita, T.; Aonuma, S.; Nagai, M.; Miyabo, A. J. Org. Chem. 1990, 55, 996. See also, Okamoto, K.; Kitagawa, T.; Takeuchi, K.; Komatsu, K.; Miyabo, A. J. Chem. Soc. Chem. Commun. 1988, 923.
211. See Alfassi, Z.B. N-Centered Radicals, Wiley, Chichester, 1998; Alfassi, Z.B. Peroxyl Radicals, Wiley, Chichester, 1997; Alfassi, Z.B. Chemical Kinetics of Small Organic Radicals, 4 Vols., CRC Press: Boca Raton, FL, 1988; Nonhebel, D.C.; Tedder, J.M.; Walton, J.C. Radicals, Cambridge University Press, Cambridge, 1979; Nonhebel, D.C.; Walton, J.C. Free-Radical Chemistry, Cambridge University Press, Cambridge, 1974; Kochi, J.K. Free Radicals, 2 Vols., Wiley, NY, 1973; Hay, J.M. Reactive Free Radicals, Academic Press, NY, 1974;. For reviews, see Kaplan, L. React. Intermed. (Wiley) 1985, 3, 227; Griller, D.; Ingold, K.U. Acc. Chem. Res. 1976, 9, 13.
212. See Dunkin, I.R. Chem. Soc. Rev. 1980, 9, 1; Jacox, M.E. Rev. Chem. Intermed. 1978, 2, 1. For a review of the study of radicals at low temperatures, see Mile, B. Angew. Chem. Int. Ed. 1968, 7, 507.
213. See. Hicks, R.G. Org. Biomol. Chem. 2007, 5, 1321. See also, Hioe, J.; Zipse, H. Org. Biomol. Chem. 2010, 8, 3609.
214. See Andrews, L. Annu. Rev. Phys. Chem. 1971, 22, 109.
215. Sullivan, P.J.; Koski, W.S. J. Am. Chem. Soc. 1963, 85, 384.
216. Sablier, M.; Fujii, T. Chem. Rev. 2002, 102, 2855.
217. Bucher, G.; Halupka, M.; Kolano, C.; Schade, O.; Sander, W. Eur. J. Org. Chem. 2001, 545.
218. See Wertz, J.E.; Bolton, J.R. Electron Spin Resonance, McGraw-Hill, NY, 1972 [reprinted by Chapman and Hall, NY, and Methuen: London, 1986]; Assenheim, H.M. Introduction to Electron Spin Resonance, Plenum, NY, 1967; Bersohn, R.; Baird, J.C. An Introduction to Electron Paramagnetic Resonance, W.A. Benjamin, NY, 1966. For reviews, see Bunce, N.J. J. Chem. Educ. 1987, 64, 907; Hirota, N.; Ohya-Nishiguchi, H. in Bernasconi, C.F. Investigation of Rates and Mechanisms of Reactions, 4th ed., pt. 2, Wiley, NY, 1986, pp. 605–655; Griller, D.; Ingold, K.U. Acc. Chem. Res. 1980, 13, 193; Norman, R.O.C. Chem. Soc. Rev. 1980, 8, 1; Fischer, H. in Kochi, J.K. Free Radicals, Vol. 2, Wiley, NY, 1973, pp. 435–491; Turro, N.J.; Kleinman, M.H.; Karatekin, E. Angew. Chem. Int. Ed. 2000, 39, 4437; Kurreck, H.; Kirste, B.; Lubitz, W. Angew. Chem. Int. Ed. 1984, 23, 173. See also, Poole, Jr., C.P. Electron Spin Resonance. A Comprehensive Treatise on Experimental Techniques, 2nd ed., Wiley, NY, 1983.
219. Davies, A.G. Chem. Soc. Rev. 1993, 22, 299.
220. See Walton, J.C. Rev. Chem. Intermed. 1984, 5, 249; Kochi, J.K. Adv. Free-Radical Chem. 1975, 5, 189; Bielski, B.H.J.; Gebicki, J.M. Atlas of Electron Spin Resonance Spectra, Academic Press, NY, 1967.
221. See Janzen, E.G.; Haire, D.L. Adv. Free Radical Chem. (Greenwich, Conn.) 1990, 1, 253; Perkins, M.J. Adv. Phys. Org. Chem. 1980, 17, 1; Zubarev, V.E.; Belevskii, V.N.; Bugaenko, L.T. Russ. Chem. Rev. 1979, 48, 729; Evans, C.A. Aldrichimica Acta 1979, 12, 23; Janzen, E.G. Acc. Chem. Res. 1971, 4, 31. See also, the collection of papers on this subject in Can. J. Chem. 1982, 60, 1379.
222. Becker, D.A.; Natero, R.; Echegoyen, L.; Lawson, R.C. J. Chem. Soc. Perkin Trans. 2 1998, 1289. Also see, Klivenyi, P.; Matthews, R.T.; Wermer, M.; Yang, L.; MacGarvey, U.; Becker, D.A.; Natero, R.; Beal, M.F. Experimental Neurobiology 1998, 152, 163.
223. For a series of papers on nitroxide radicals, see Pure Appl. Chem. 1990, 62, 177.
224. Janzen, E.G.; Zhang, Y.-K. J. Org. Chem. 1995, 60, 5441. For the preparation of a new but structurally related spin trap see Karoui, H.; Nsanzumuhire, C.; Le Moigne, F.; Tordo, P. J. Org. Chem. 1999, 64, 1471.
225. Grossi, L.; Strazzari, S. Chem. Commun. 1997, 917.
226. Timberlake, J.W.; Chen, T. Tetrahedron Lett. 1994, 35, 6043; Tanko, J.M.; Brammer, Jr., L.E.; Hervas', M.; Campos, K. J. Chem. Soc. Perkin Trans. 2 1994, 1407.
227. Harry Frank, University of Connecticut, Storrs, CT., Personal Communication.
228. Ward, H.R.; Lawler, R.G.; Cooper, R.A. J. Am. Chem. Soc. 1969, 91, 746; Lepley, A.R. J. Am. Chem. Soc. 1969, 91, 749; Lepley, A.R.; Landau, R.L. J. Am. Chem. Soc. 1969, 91, 748.
229. See Lepley, R.L.; Closs, G.L. Chemically Induced Magnetic Polarization, Wiley, NY, 1973. Bargon, J. Helv. Chim. Acta 2006, 89, 2082. For reviews, see Adrian, F.J. Rev. Chem. Intermed. 1986, 7, 173; Closs, G.L.; Miller, R.J.; Redwine, O.D. Acc. Chem. Res. 1985, 18, 196; Closs, G.L. Adv. Magn. Reson. 1974, 7, 157; Lawler, R.G. Acc. Chem. Res. 1972, 5, 25; Kaptein, R. Adv. Free-Radical Chem. 1975, 5, 319.
230. A related technique is called CIDEP. For a review, see Hore, P.J.; Joslin, C.G.; McLauchlan, K.A. Chem. Soc. Rev. 1979, 8, 29.
231. Ward, H.R.; Lawler, R.G.; Cooper, R.A. J. Am. Chem. Soc. 1969, 91, 746.
232. It has been shown that CIDNP can also arise in cases where para hydrogen (H2 in which the nuclear spins are opposite) is present: Eisenschmid, T.C.; Kirss, R.U.; Deutsch, P.P.; Hommeltoft, S.I.; Eisenberg, R.; Bargon, J.; Lawler, R.G.; Balch, A.L. J. Am. Chem. Soc. 1987, 109, 8089.
233. Wind, R.A.; Duijvestijn, M.J.; van der Lugt, C.; Manenschijn, A.; Vriend, J. Prog. Nucl. Magn. Reson. Spectrosc. 1985, 17, 33.
234. Hu, K.-N.; Yu, H.-h.; Swager, T. M.; Griffin, R. G. J. Am. Chem. Soc. 2004, 126, 10844. A discussion of electronic effects is found in Wagner, P.J.; Wang, L. Org. Lett. 2006, 8, 645.
235. For a discussion of the role of alkyl substitution with respect to radical stabilization, see Gronert, S. J. Org. Chem. 2006, 71, 7045. For a discussion concerning data that hyperconguation stabilizes alkyl radicals, see Gronert, S. Org. Lett. 2007, 9, 2211.
236. For a discussion, see Robaugh, D.A.; Stein, S.E. J. Am. Chem. Soc. 1986, 108, 3224.
237. See Forrester, A.R.; Hay, J.M.; Thomson, R.H. Organic Chemistry of Stable Free Radicals, Academic Press, NY, 1968.
238. For an electron diffraction study of the allyl radical, see Vajda, E.; Tremmel, J.; Rozsondai, B.; Hargittai, I.; Maltsev, A.K.; Kagramanov, N.D.; Nefedov, O.M. J. Am. Chem. Soc. 1986, 108, 4352.
239. Asensio, A.; Dannenberg, J. J. J. Org. Chem. 2001, 66, 5996.
240. For a review, see Sholle, V.D.; Rozantsev, E.G. Russ. Chem. Rev. 1973, 42, 1011.
241. Gomberg, M. J. Am. Chem. Soc. 1900, 22, 757, Ber. 1900, 33, 3150.
242. For hexaphenylethane derivatives, see Stein, M.; Winter, W.; Rieker, A. Angew. Chem. Int. Ed. 1978, 17, 692; Yannoni, N.; Kahr, B.; Mislow, K. J. Am. Chem. Soc. 1988, 110, 6670.
243. Volz, H.; Lotsch, W.; Schnell, H. Tetrahedron 1970, 26, 5343; McBride, J. Tetrahedron 1974, 30, 2009. See Guthrie, R.D.; Weisman, G.R. Chem. Commun. 1969, 1316; Takeuchi, H.; Nagai, T.; Tokura, N. Bull. Chem. Soc. Jpn. 1971, 44, 753; Peyman, A.; Peters, K.; von Schnering, H.G.; Rüchardt, C. Chem. Ber. 1990, 123, 1899.
244. For a review of steric effects in free radical chemistry, see Rüchardt, C. Top. Curr. Chem. 1980, 88, 1.
245. Sabacky, M.J.; Johnson, Jr., C.S.; Smith, R.G.; Gutowsky, H.S.; Martin, J.C. J. Am. Chem. Soc. 1967, 89, 2054.
246. Müller, E.; Moosmayer, A.; Rieker, A.; Scheffler, K. Tetrahedron Lett. 1967, 3877. See also, Neugebauer, F.A.; Hellwinkel, D.; Aulmich, G. Tetrahedron Lett. 1978, 4871.
247. Kaba, R.A.; Ingold, K.U. J. Am. Chem. Soc. 1976, 98, 523.
248. Zarkadis, A.K.; Neumann, W.P.; Marx, R.; Uzick, W. Chem. Ber. 1985, 118, 450; Zarkadis, A.K.; Neumann, W.P.; Uzick, W. Chem. Ber. 1985, 118, 1183.
249. Dünnebacke, D.; Neumann, W.P.; Penenory, A.; Stewen, U. Chem. Ber. 1989, 122, 533.
250. For reviews, see Ballester, M. Adv. Phys. Org. Chem. 1989, 25, 267, pp. 354–405; Acc. Chem. Res. 1985, 18, 380. See also, Hegarty, A.F.; O'Neill, P. Tetrahedron Lett. 1987, 28, 901.
251. Fort, Jr., R.C.; Hrovat, D.A.; Borden, W.T. J. Org. Chem. 1993, 58, 211.
252. Galli, C.; Guarnieri, A.; Koch, H.; Mencarelli, P.; Rappoport, Z. J. Org. Chem. 1997, 62, 4072.
253. Rogers, D.W.; Matsunaga, N.; Zavitsas, A.A. J. Org. Chem. 2006, 71, 2214.
254. Gottschling, S.E.; Grant, T.N.; Milnes, K.K.; Jennings, M.C.; Baines, K.M. J. Org. Chem. 2005, 70, 2686.
255. Giese, B.; Damm, W.; Wetterich, F.; Zeltz, H.-G.; Rancourt, J.; Guindon, Y. Tetrahedron Lett. 1993, 34, 5885.
256. For reviews, see Sustmann, R.; Korth, H. Adv. Phys. Org. Chem. 1990, 26, 131; Viehe, H.G.; Janousek, Z.; Merényi, R.; Stella, L. Acc. Chem. Res. 1985, 18, 148.
257. See Pasto, D.J. J. Am. Chem. Soc. 1988, 110, 8164. See also, Ashby, E.C. Bull. Soc. Chim. Fr. 1972, 2133; Bell, N.A. Educ. Chem. 1973, 143.
258. See Sakurai, H.; Kyushin, S.; Nakadaira, Y.; Kira, M. J. Phys. Org. Chem. 1988, 1, 197; Rhodes, C.J.; Roduner, E. Tetrahedron Lett. 1988, 29, 1437; Viehe, H.G.; Merényi, R.; Janousek, Z. Pure Appl. Chem. 1988, 60, 1635; Bordwell, F.G.; Lynch, T. J. Am. Chem. Soc. 1989, 111, 7558.
259. See Bordwell, F.G.; Bausch, M.J.; Cheng, J.P.; Cripe, T.H.; Lynch, T.-Y.; Mueller, M.E. J. Org. Chem. 1990, 55, 58; Bordwell, F.G.; Harrelson, Jr., J.A. Can. J. Chem. 1990, 68, 1714.
260. See Pasto, D.J. J. Am. Chem. Soc. 1988, 110, 8164.
261. Jiang, X.; Li, X.; Wang, K. J. Org. Chem. 1989, 54, 5648.
262. For reviews of radicals with the unpaired electron on atoms other than carbon, see, in Kochi, J.K. Free Radicals, Vol. 2, Wiley, NY, 1973, the reviews by Nelson, S.F. pp. 527–593 (N-centered); Bentrude, W.G. pp. 595–663 (P-centered); Kochi, J.K. pp. 665–710 (O-centered); Kice, J.L. pp. 711–740 (S-centered); Sakurai, H. pp. 741–807 (Si, Ge, Sn, and Pb centered).
263. Maki, T.; Araki, Y.; Ishida, Y.; Onomura, O.; Matsumura, Y. J. Am. Chem. Soc. 2001, 123, 3371.
264. Jeromin, G.E. Tetrahedron Lett. 2001, 42, 1863.
265. See Novak, I.; Harrison, L.J.; Kova![]() , B.; Pratt, L.M. J. Org. Chem. 2004, 69, 7628.
, B.; Pratt, L.M. J. Org. Chem. 2004, 69, 7628.
266. See Anelli, P.L.; Montanari, F.; Quici, S. Org. Synth. 1990, 69, 212; Fritz-Langhals, E. Org. Process Res. Dev. 2005, 9, 577. See also, Rychnovsky, S.D.; Vaidyanathan, R.; Beauchamp, T.; Lin, R.; Farmer, P.J. J. Org. Chem.1999, 64, 6745.
267. Volodarsky, L.B.; Reznikov, V.A.; Ovcharenko, V.I. Synthetic Chemistry of Stable Nitroxides, CRC Press, Boca Raton, FL, 1994; Keana, J.F.W. Chem. Rev. 1978, 78, 37; Aurich, H.G. Nitroxides. In Nitrones, Nitronates, Nitroxides, Patai, S.; Rappoport, Z., Eds., Wiley, NY, 1989; Chap. 4.
268. Neiman, M.B.; Rozantsev, E.G.; Mamedova, Yu.G. Nature (London) 1963, 200, 256. See Breuer, E.; Aurich, H.G.; Nielsen, A. Nitrones, Nitronates, and Nitroxides, Wiley, NY, 1989, pp. 313–399; Rozantsev, E.G.; Sholle, V.D. Synthesis 1971, 190, 401.
269. See Ballester, M.; Veciana, J.; Riera, J.; Castañer, J.; Armet, O.; Rovira, C. J. Chem. Soc. Chem. Commun. 1983, 982.
270. Adam, W.; Ortega Schulte, C. M. J. Org. Chem. 2002, 67, 4569.
271. Miura, Y.; Matsuba, N.; Tanaka, R.; Teki, Y.; Takui, T. J. Org. Chem. 2002, 67, 8764. For another stable nitroxide radical, see Huang, W.-l.; Chiarelli, R.; Rassat, A. Tetrahedron Lett. 2000, 41, 8787.
272. Miura, Y.; Tomimura, T.; Matsuba, N.; Tanaka, R.; Nakatsuji, M.; Teki, Y. J. Org. Chem. 2001, 66, 7456. See also, Miura, Y.; Muranaka, Y.; Teki, Y. J. Org. Chem. 2006, 71, 4786; Miura, Y.; Mu, Y. Chem. Lett. 2005, 34, 48
273. Janzen, E.G.; Chen, G.; Bray, T.M.; Reinke, L.A.; Poyer, J.L.; McCay, P.B. J. Chem. Soc. Perkin Trans. 2. 1993, 1983.
274. Reznikov, V.A.; Volodarsky, L.B. Tetrahedron Lett. 1994, 35, 2239.
275. Reznikov, V.A.; Pervukhina, N.V.; Ikorskii, V.N.; Ovcharenko, V.I..; Grand, A. Chem. Commun. 1999, 539.
276. Apeloig, Y.; Bravo-Zhivotovskii, D.; Bendikov, M.; Danovich, D.; Botoshansky, M.; Vakulrskaya, T.; Voronkov, M.; Samoilova, R.; Zdravkova, M.; Igonin, V.; Shklover, V.; Struchkov, Y. J. Am. Chem. Soc. 1999, 121, 8118.
277. It has been claimed that relative D values do not provide such a measure: Nicholas, A.M. de P.; Arnold, D.R. Can. J. Chem. 1984, 62, 1850, 1860.
278. Except where noted, these values are from Lide, D.R. (Ed.), Handbook of Chemistry and Physics, 87th ed.; CRC Press: Boca Raton, FL, 2007, pp. 9-60–9-61. For another list of D values, see McMillen, D.F.; Golden, D.M. Annu. Rev. Phys. Chem. 1982, 33, 493. See also, Holmes, J.L.; Lossing, F.P.; Maccoll, A. J. Am. Chem. Soc. 1988, 110, 7339; Holmes, J.L.; Lossing, F.P. J. Am. Chem. Soc. 1988, 110, 7343; Roginskii, V.A. J. Org. Chem. USSR1989, 25, 403.
279. For the IR of a matrix-isolated phenyl radical, see Friderichsen, A. V.; Radziszewski, J. G.; Nimlos, M. R.; Winter, P. R.; Dayton, D. C.; David, D. E.; Ellison, G. B. J. Am. Chem. Soc. 2001, 123, 1977.
280. For a review of cyclopropyl radicals, see Walborsky, H.M. Tetrahedron 1981, 37, 1625. See also, Boche, G.; Walborsky, H.M. Cyclopropane Derived Reactive Intermediates, Wiley, NY, 1990.
281. This value is from Gutman, D. Acc. Chem. Res. 1990, 23, 375.
282. Zhang, X.-M. J. Org. Chem. 1998, 63, 1872.
283. Brocks, J.J.; Beckhaus, H.-D.; Beckwith, A.L.J.; Rüchardt, C. J. Org. Chem. 1998, 63, 1935.
284. Pratt, D.A.; Porter, N.A. Org. Lett. 2003, 5, 387.
285. Zavitsas, A.A.; Rogers, D.W.; Matsunaga, N. J. Org. Chem. 2010, 75, 5697.
286. For a review, see Kaplan, L. in Kochi, J.K. Free Radicals, Vol. 2, Wiley, NY, 1973, pp. 361–434.
287. See Giese, B.; Beckhaus, H. Angew. Chem. Int. Ed. 1978, 17, 594; Ellison, G.B.; Engelking, P.C.; Lineberger, W.C. J. Am. Chem. Soc. 1978, 100, 2556. See, however, Paddon-Row, M.N.; Houk, K.N. J. Am. Chem. Soc. 1981, 103, 5047.
288. There are a few exceptions. See Section 14.A.iv.
289. Herzberg, G. Proc. R. Soc. London, Ser. A 1961, 262, 291. See also, Tan, L.Y.; Winer, A.M.; Pimentel, G.C. J. Chem. Phys. 1972, 57, 4028; Yamada, C.; Hirota, E.; Kawaguchi, K. J. Chem. Phys. 1981, 75, 5256.
290. Andrews, L.; Pimentel, G.C. J. Chem. Phys. 1967, 47, 3637; Milligan, D.E.; Jacox, M.E. J. Chem. Phys. 1967, 47, 5146.
291. Tamura, R.; Susuki, S.; Azuma, N.; Matsumoto, A.; Todda, F.; Ishii, Y. J. Org. Chem. 1995, 60, 6820.
292. Rychnovsky, S.D.; Powers, J.P.; LePage, T.J. J. Am. Chem. Soc. 1992, 114, 8375.
293. Danen, W.C.; Tipton, T.J.; Saunders, D.G. J. Am. Chem. Soc. 1971, 93, 5186; Fort, Jr., R.C.; Hiti, J. J. Org. Chem. 1977, 42, 3968; Lomas, J.S. J. Org. Chem. 1987, 52, 2627.
294. Fessenden, R.W.; Schuler, R.H. J. Chem. Phys. 1965, 43, 2704; Rogers, M.T.; Kispert, L.D. J. Chem. Phys. 1967, 46, 3193; Pauling, L. J. Chem. Phys. 1969, 51, 2767.
295. See Chen, K.S.; Tang, D.Y.H.; Montgomery, L.K.; Kochi, J.K. J. Am. Chem. Soc. 1974, 96, 2201. For a discussion, see Krusic, P.J.; Bingham, R.C. J. Am. Chem. Soc. 1976, 98, 230.
296. See Deycard, S.; Hughes, L.; Lusztyk, J.; Ingold, K.U. J. Am. Chem. Soc. 1987, 109, 4954.
297. Adrian, F.J. J. Chem. Phys. 1958, 28, 608; Andersen, P. Acta Chem. Scand. 1965, 19, 629.
298. Kubota, S.; Matsushita, M.; Shida, T.; Abu-Raqabah, A.; Symons, M.C.R.; Wyatt, J.L. Bull. Chem. Soc. Jpn. 1995, 68, 140.
299. See Borden, W.T. Diradicals, Wiley, NY, 1982; Johnston, L.J.; Scaiano, J.C. Chem. Rev. 1989, 89, 521; Doubleday Jr., C.; Turro, N.J.; Wang, J. Acc. Chem. Res. 1989, 22, 199; Scheffer, J.R.; Trotter, J. Rev. Chem. Intermed.1988, 9, 271; Wilson, R.M. Org. Photochem. 1985, 7, 339; Borden, W.T. React. Intermed. (Wiley) 1985, 3, 151; 1981, 2, 175; Borden, W.T.; Davidson, E.R. Acc. Chem. Res. 1981, 14, 69. See also, Döhnert, D.; Koutecky, J. J. Am. Chem. Soc. 1980, 102, 1789. For a series of papers on diradicals, see Tetrahedron 1982, 38, 735. For a stable hydrocarbon diradical, see Rajca, A.; Shiraishi, K.; Vale, M.; Han, H.; Rajca, S. J. Am. Chem. Soc. 2005, 127, 9014.
300. Zhang, D. Y.; Borden, W. T. J. Org. Chem. 2002, 67, 3989.
301. Ma, J.; Ding, Y.; Hattori, K.; Inagaki, S. J. Org. Chem. 2004, 69, 4245
302. For reviews of trimethylenemethane, see Borden, W.T.; Davidson, E.R. Ann. Rev. Phys. Chem. 1979, 30, 125; Bergman, R.G. in Kochi, J.K. Free Radicals, Vol. 1, Wiley, NY, 1973, pp. 141–149.
303. See Turro, N.J. J. Chem. Educ. 1969, 46, 2; Wasserman, E.; Hutton, R.S. Acc. Chem. Res. 1977, 10, 27; Ichinose, N.; Mizuno, K.; Otsuji, Y.; Caldwell, R.A.; Helms, A.M. J. Org. Chem. 1998, 63, 3176.
304. Matsuda, K.; Iwamura, H. J. Chem. Soc. Perkin Trans. 2 1998, 1023. Also see, Roth, W.R.; Wollweber, D.; Offerhaus, R.; Rekowski, V.; Lenmartz, H.-W.; Sustmann, R.; Müller, W. Chem. Ber. 1993, 126, 2701.
305. Inoue, K.; Iwamura, H. Angew. Chem. Int. Ed. 1995, 34, 927. Also see, Ulrich, G.; Ziessel, R.; Luneau, D.; Rey, P. Tetrahedron Lett. 1994, 35, 1211.
306. Engel, P.S.; Lowe, K.L. Tetrahedron Lett. 1994, 35, 2267.
307. Liao, Y.; Xie, C.; Lahti, P.M.; Weber, R.T.; Jiang, J.; Barr, D.P. J. Org. Chem. 1999, 64, 5176.
308. Cai, X.; Cygon, P.; Goldfuss, B.; Griesbeck, A.G.; Heckroth, H.; Fujitsuka, M.; Majima, T. Chemistry: European J. 2006, 12, 4662.
309. See Giese, B. Radicals in Organic Synthesis: Formation of Carbon–Carbon Bonds, Pergamon, Elmsford, NY, 1986, pp. 267–281; Brown, R.F.C. Pyrolytic Methods in Organic Chemistry, Academic Press, NY, 1980, pp. 44–61.
310. See Harmony, J.A.K. Methods Free-Radical Chem. 1974, 5, 101.
311. See Barker, P.J.; Winter, J.N. in Hartley, F.R.; Patai, S. The Chemistry of the Metal–Carbon Bond, Vol. 2, Wiley, NY, 1985, pp. 151–218.
312. Matsuyama, K.; Sugiura, T.; Minoshima, Y. J. Org. Chem. 1995, 60, 5520; Ryzhkov, L.R. J. Org. Chem. 1996, 61, 2801. See Howard, J.A. in Patai, S. The Chemistry of Peroxides, Wiley, NY, 1983, pp. 235–258; Batt, L.; Liu, M.T.H. in the same volume, pp. 685–710.
313. See Engel, P.S. Chem. Rev. 1980, 80, 99; Adams, J.S.; Burton, K.A.; Andrews, B.K.; Weisman, R.B.; Engel, P.S. J. Am. Chem. Soc. 1986, 108, 7935; Schmittel, M.; Rüchardt, C. J. Am. Chem. Soc. 1987, 109, 2750.
314. Cossy, J.; Ranaivosata, J.-L.; Bellosta, V. Tetrahedron Lett. 1994, 35, 8161.
315. Courtneidge, J.L. Tetrahedron Lett. 1992, 33, 3053.
316. Pasto, D.J.; Cottard, F. Tetrahedron Lett. 1994, 35, 4303.
317. Halliwell, B.; Gutteridge, J.M.C. in Free Radicals in Biology and Medicine, Oxford University Press, Oxford, 1999, pp 246–350; DeMatteo, M.P.; Poole, J.S.; Shi, X.; Sachdeva, R.; Hatcher, P.G.; Hadad, C.M.; Platz, M.S. J. Am. Chem. Soc. 2005, 127, 7094.
318. Johnston, L.J. Chem. Rev. 1993, 93, 251.
319. See Costentin, C.; Robert, M.; Saveant, J.-M. J. Am. Chem. Soc. 2003, 125, 105.
320. See Pilling, M.J. Int. J. Chem. Kinet. 1989, 21, 267; Khudyakov, I.V.; Levin, P.P.; Kuz'min, V.A. Russ. Chem. Rev. 1980, 49, 982; Gibian, M.J.; Corley, R.C. Chem. Rev. 1973, 73, 441.
321. Cuerva, J.M.; Campaña, A.G.; Justicia, J.; Rosales, A.; Oller-López, J.L.; Robles, R.; Cárdenas, D.J.; Buñuel, E.; Oltra, J.E. Angew. Chem. Int. Ed. 2006, 45, 5522.
322. Hammerum, S. J. Am. Chem. Soc. 2009, 131, 8627.
323. Dolenc, D.; Plesniar, B. J. Org. Chem. 2006, 71, 8028.
324. Bietti, M.; Salamone, M. Org. Lett. 2010, 12, 3654.
325. See Stevenson, J. P.; Jackson, W. F.; Tanko, J. M. J. Am. Chem. Soc. 2002, 124, 4271.
326. LeTadic-Biadatti, M.-H.; Newcomb, M. J. Chem. Soc. Perkin Trans. 2 1996, 1467. See also, Choi, S.-Y.; Horner, J. H.; Newcomb, M. J. Org. Chem. 2000, 65, 4447; Cooksy, A. L.; King, H. F.; Richardson, W. H. J. Org. Chem. 2003, 68, 9441; Tian, F.; Dolbier, Jr., W.R. Org. Lett. 2000, 2, 835.
327. Halgren, T. A.; Roberts, J. D.; Horner, J. H.; Martinez, F. N.; Tronche, C.; Newcomb, M. J. Am. Chem. Soc. 2000, 122, 2988.
328. Newcomb, M.; Choi, S.-Y.; Toy, P. H. Can. J. Chem. 1999, 77, 1123; Nevill, S. M.; Pincock, J. A. Can. J. Chem. 1997, 75, 232.
329. See Barton, D.H.R.; Jacob, M.; Peralez, E. Tetrahedron Lett. 1999, 40, 9201.
330. Choi, S.-Y.; Horner, J.H.; Newcomb, M. J. Org. Chem. 2000, 65, 4447; Engel, P.S.; He, S.-L.; Banks, J.T.; Ingold, K.U.; Lusztyk, J. J. Org. Chem. 1997, 62, 1210.
331. See Leardini, R.; Lucarini, M.; Pedulli, G.F.; Valgimigli, L. J. Org. Chem. 1999, 64, 3726; Roschek, Jr., B.; Tallman, K.A.; Rector, C.L.; Gillmore, J.G.; Pratt, D.A.; Punta, C.; Porter, N.A. J. Org. Chem. 2006, 71, 3527.
332. See Khudyakov, I.V.; Kuz'min, V.A. Russ. Chem. Rev. 1978, 47, 22.
333. See Kaiser, E.T.; Kevan, L. Radical Ions, Wiley, NY, 1968; Gerson, F.; Huber, W. Acc. Chem. Res. 1987, 20, 85; Todres, Z.V. Tetrahedron 1985, 41, 2771; Holy, N.L.; Marcum, J.D. Angew. Chem. Int. Ed. 1971, 10, 115. See Chanon, M.; Rajzmann, M.; Chanon, F. Tetrahedron 1990, 46, 6193. For a series of papers on this subject, see Tetrahedron 1986, 42, 6097.
334. See Depew, M.C.; Wan, J.K.S. in Patai, S.; Rappoport, Z. The Chemistry of the Quinonoid Compounds, Vol. 2, pt. 2, Wiley, NY, 1988, pp. 963–1018; Huh, C.; Kang, C.H.; Lee, H.W.; Nakamura, H.; Mishima, M.; Tsuno, Y.; Yamataka, H. Bull. Chem. Soc. Jpn. 1999, 72, 1083.
335. de Meijere, A.; Gerson, F.; Schreiner, P.R.; Merstetter, P.; Schüngel, F.-M. Chem. Commun. 1999, 2189.
336. See Russell, G.A. in Patai, S.; Rappoport, Z. The Chemistry of Enones, pt. 1, Wiley, NY, 1989, pp. 471–512. See Davies, A.G.; Neville, A.G. J. Chem. Soc. Perkin Trans. 2 1992, 163, 171.
337. Ishida, S.; Iwamoto, T.; Kira, M. J. Am. Chem. Soc. 2003, 125, 3212; Sekiguchi, A.; Tanaka, T.; Ichinohe, M.; Akiyama, K.; Tero-Kubota, S. J. Am. Chem. Soc. 2003, 125, 4962; Inoue, S.; Ichinohe, M.; Sekiguchi, A. J. Am. Chem. Soc. 2007, 129, 6096.
338. Villano, S.M.; Eyet, N.; Lineberger, W.C.; Bierbaum, V.M. J. Am. Chem. Soc. 2008, 130, 7214.
339. See Roth, H.D. Acc. Chem. Res. 1987, 20, 343; Courtneidge, J.L.; Davies, A.G. Acc. Chem. Res. 1987, 20, 90; Symons, M.C.R. Chem. Soc. Rev. 1984, 13, 393; Marchetti, F.; Pinzino, C.; Zacchini. S.; Guido, G. Angew. Chem. Int. Ed. 2010, 49, 5268.
340. Gerson, F.; Scholz, M.; Hansen, H.-J.; Uebelhart, P. J. Chem. Soc. Perkin Trans. 2 1995, 215.
341. de Meijere, A.; Chaplinski, V.; Gerson, F.; Merstetter, P.; Haselbach, E. J. Org. Chem. 1999, 64, 6951.
342. Neugebauer, F.A.; Funk, B.; Staab, H.A. Tetrahedron Lett. 1994, 35, 4755. See Stickley, K.R.; Blackstock, S.C. Tetrahedron Lett. 1995, 36, 1585.
343. Dauben, W.G.; Cogen, J.M.; Behar, V.; Schultz, A.G.; Geiss, W.; Taveras, A.G. Tetrahedron Lett. 1992, 33, 1713.
344. Rhodes, C.J.; AgirBas H. J. Chem. Soc. Perkin Trans. 2 1992, 397.
345. Gerson, F.; Felder, P.; Schmidlin, R.; Wong, H.N.C. J. Chem. Soc. Chem. Commun. 1994, 1659.
346. Wartini, A.R.; Valenzuela, J.; Staab, H.A.; Neugebauer, F.A. Eur. J. Org. Chem. 1998, 139.
347. Nelson, S.F.; Reinhardt, L.A.; Tran, H.Q.; Clark, T.; Chen, G.-F.; Pappas, R.S.; Williams, F. Chem. Eur. J. 2002, 8, 1074.
348. See Jones, Jr., M.; Moss, R.A. Carbenes, 2 Vols., Wiley, NY, 1973–1975; Rees, C.W.; Gilchrist, T.L. Carbenes, Nitrenes, and Arynes, Nelson, London, 1969; Minkin, V.I.; Simkin, B.Ya.; Glukhovtsev, M.N. Russ. Chem. Rev.1989, 58, 622; Moss, R.A.; Jones, Jr., M. React. Intermed. (Wiley) 1985, 3, 45; Liebman, J.F.; Simons, J. Mol. Struct. Energ. 1986, 1, 51.
349. See Nefedov, O.M.; Maltsev, A.K.; Mikaelyan, R.G. Tetrahedron Lett. 1971, 4125; Wright, B.B. Tetrahedron 1985, 41, 1517. For reviews, see Zuev, P.S.; Nefedov, O.M. Russ. Chem. Rev. 1989, 58, 636; Sheridan, R.S. Org. Photochem. 1987, 8, 159, pp. 196–216; Trozzolo, A.M. Acc. Chem. Res. 1968, 1, 329.
350. Skell, P.S. Tetrahedron 1985, 41, 1427.
351. See Closs, G.L. Top. Stereochem. 1968, 3, 193, pp. 203–210; Bethell, D. Adv. Phys. Org. Chem. 1969, 7, 153, p. 194; Hoffmann, R. J. Am. Chem. Soc. 1968, 90, 1475.
352. Richards, Jr., C.A.; Kim, S.-J.; Yamaguchi, Y.; Schaefer, III, H.F. J. Am. Chem. Soc. 1995, 117, 10104.
353. See Lengel, R.K.; Zare, R.N. J. Am. Chem. Soc. 1978, 100, 7495; Borden, W.T.; Davidson, E.R. Ann. Rev. Phys. Chem. 1979, 30, 125, see pp. 128–134; Leopold, D.G.; Murray, K.K.; Lineberger, W.C. J. Chem. Phys. 1984, 81, 1048.
354. Kopecky, K.R.; Hammond, G.S.; Leermakers, P.A. J. Am. Chem. Soc. 1961, 83, 2397; 1962, 84, 1015; Duncan, F.J.; Cvetanovi![]() , R.J. J. Am. Chem. Soc. 1962, 84, 3593.
, R.J. J. Am. Chem. Soc. 1962, 84, 3593.
355. For a review of the kinetics of CH2 reactions, see Laufer, A.H. Rev. Chem. Intermed. 1981, 4, 225.
356. See Turro, N.J.; Cha, Y.; Gould, I.R. J. Am. Chem. Soc. 1987, 109, 2101.
357. Tomioka, H. Acc. Chem. Res. 1997, 30, 315; Kirmse, W. Angew. Chem. Int. Ed. 2003, 42, 2117; Hirai, K.; Itoh, T.; Tomioka, H. Chem. Rev. 2009, 109, 3275.
358. Woodcock, H.L.; Moran, D.; Schleyer, P.v.R.; Schaefer, III, H.F. J. Am. Chem. Soc. 2001, 123, 4331.
359. Itoh, T.; Nakata, Y.; Hirai, K.; Tomioka, H. J. Am. Chem. Soc. 2006, 128, 957.
360. Cattoën, X.; Miqueu, K.; Gornitzka, H.; Bourissou, D.; Bertrand, G. J. Am. Chem. Soc. 2005, 127, 3292.
361. For other methods of distinguishing singlet from triplet carbenes, see Hendrick, M.E.; Jones, Jr., M. Tetrahedron Lett. 1978, 4249; Creary, X. J. Am. Chem. Soc. 1980, 102, 1611.
362. Rabinovitch, B.S.; Tschuikow-Roux, E.; Schlag, E.W. J. Am. Chem. Soc. 1959, 81, 1081; Frey, H.M. Proc. R. Soc. London, Ser. A 1959, 251, 575; Lambert, J.B.; Larson, E.G.; Bosch, R.J. Tetrahedron Lett. 1983, 24, 3799.
363. Andrews, L. J. Chem. Phys. 1968, 48, 979.
364. The technique of spin trapping (Sec. 5.C.i) has been applied to the detection of transient triplet carbenes: Forrester, A.R.; Sadd, J.S. J. Chem. Soc. Perkin Trans. 2 1982, 1273.
365. Wasserman, E.; Kuck, V.J.; Hutton, R.S.; Anderson, E.D.; Yager, W.A. J. Chem. Phys. 1971, 54, 4120; Bernheim, R.A.; Bernard, H.W.; Wang, P.S.; Wood, L.S.; Skell, P.S. J. Chem. Phys. 1971, 54, 3223.
366. Hahn, F.E. Angew. Chem. Int. Ed. 2006, 45, 1348. For imidazopyridine carbenes, see Moss, R.A.; Tian, J.; Sauers, R.R.; Krogh-Jespersen, K. J. Am. Chem. Soc. 2007, 129, 10019.
367. Herzberg, G.; Johns, J.W.C. J. Chem. Phys. 1971, 54, 2276 and cited references.
368. Ivey, R.C.; Schulze, P.D.; Leggett, T.L.; Kohl, D.A. J. Chem. Phys. 1974, 60, 3174.
369. Senthilnathan, V.P.; Platz, M.S. J. Am. Chem. Soc. 1981, 103, 5503; Gilbert, B.C.; Griller, D.; Nazran, A.S. J. Org. Chem. 1985, 50, 4738.
370. For reviews of halocarbenes, see Burton, D.J.; Hahnfeld, J.L. Fluorine Chem. Rev. 1977, 8, 119; Margrave, J.L.; Sharp, K.G.; Wilson, P.W. Fort. Chem. Forsch. 1972, 26, 1, pp. 3–13.
371. See Stang, P.J. Acc. Chem. Res. 1982, 15, 348; Chem. Rev. 1978, 78, 383; Marchand, A.P.; Brockway, N.M. Chem. Rev. 1974, 74, 431; Schuster, G.B. Adv. Phys. Org. Chem. 1986, 22, 311. For a review of carbenes with neighboring hetero atoms, see Taylor, K.G. Tetrahedron 1982, 38, 2751.
372. Alcarazo, M.; Roseblade, S.J.; Cowley, A.R.; Fernández, R.; Brown, J.M.; Lassaletta, J.M. J Am. Chem. Soc. 2005, 127, 3290. See also, Kassaee, M.Z.; Shakib, F.A.; Momeni, M.R.; Ghambarian, M.; Musavi, S.M. J. Org. Chem. 2010, 75, 2539.
373. Krahulic, K.E.; Enright, G.D.; Parvez, M.; Roesler, R. J. Am. Chem. Soc. 2005, 127, 4142.
374. Herrmann, W.A. Angew. Chem. Int. Ed. 2002, 41, 1290.
375. Ye, Q.; Komarov, I. V.; Kirby, A. J.; Jones, Jr., M. J. Org. Chem. 2002, 67, 9288.
376. Ye, Q.; Jones Jr., M.; Chen, T.; Shevlin, P.B. Tetrahedron Lett. 2001, 42, 6979.
377. Ohira, S.; Yamasaki, K.; Nozaki, H.; Yamato, M.; Nakayama, M. Tetrahedron Lett. 1995, 36, 8843. For dimethylvinylidene carbene see Reed, S.C.; Capitosti, G.J.; Zhu, Z.; Modarelli, D.A. J. Org. Chem. 2001, 66, 287. For a review of akylidenecarbenes, see Knorr, R. Chem. Rev. 2004, 104, 3795.
378. Fernamberg, K.; Snoonian, J.R.; Platz, M.S. Tetrahedron Lett. 2001, 42, 8761.
379. Creary, X.; Butchko, M.A. J. Org. Chem. 2002, 67, 112.
380. Bonnichon, F.; Richard, C.; Grabner, G. Chem. Commun. 2001, 73.
381. Zuev, P. S.; Sheridan, R. S. J. Am. Chem. Soc. 2004, 126, 12220.
382. Topolski, M.; Duraisamy, M.; Racho![]() , J.; Gawronski, J.; Gawronska, K.; Goedken, V.; Walborsky, H.M. J. Org. Chem. 1993, 58, 546.
, J.; Gawronski, J.; Gawronska, K.; Goedken, V.; Walborsky, H.M. J. Org. Chem. 1993, 58, 546.
383. Kirmse, W. Angew. Chem. Int. Ed. 2005, 44, 2476.
384. See Wanzlick, H.-W.; Schikora, E. Angew. Chem. 1960, 72, 494.
385. Ruzsicska, B.P.; Jodhan, A.; Choi, H.K.J.; Strausz, O.P. J. Am. Chem. Soc. 1983, 105, 2489.
386. Zeidan, T.A.; Kovalenko, S.V.; Manoharan, M.; Clark, R.J.; Ghiviriga, I.; Alabugin, I.V. J. Am. Chem. Soc. 2005, 127, 4270.
387. See Jones, Jr., M. Acc. Chem. Res. 1974, 7, 415; Kirmse, W. in Bamford, C.H.; Tipper, C.F.H. Comprehensive Chemical Kinetics, Vol. 9; Elsevier, NY, 1973, pp. 373–415; Ref. 348; Petrosyan, V.E.; Niyazymbetov, M.E. Russ. Chem. Rev. 1989, 58, 644.
388. For a review of formation of carbenes in this manner, see Kirmse, W. Angew. Chem. Int. Ed. 1965, 4, 1.
389. Ashby, E.C.; Deshpande, A.K.; Doctorovich, F. J. Org. Chem. 1993, 58, 4205. For a preparation from diclorodiazirine, see Chu, G.; Moss, R.A.; Sauers, R.R. J. Am. Chem. Soc. 2005, 127, 14206. Also see Moss, R.A.; Tian, J.; Sauers, R.R.; Ess, D.H.; Houk, K.N.; Krogh-Jespersen, K. J. Am. Chem. Soc. 2007, 129, 5167.
390. Wagner, W.M. Proc. Chem. Soc. 1959, 229.
391. Glick, H.C.; Likhotvovik, I.R.; Jones, Jr., M. Tetrahedron Lett. 1995, 36, 5715; Stang, P.J. Acc. Chem. Res. 1982, 15, 348; Chem. Rev. 1978, 78, 383.
392. For a review, see Regitz, M.; Maas, G. Diazo Compounds, Academic Press, NY, 1986, pp. 170–184.
393. For example, see Mieusset, J.-L.; Brinker, U.H. J. Org. Chem. 2006, 71, 6975.
394. See Martinu, T.; Dailey, W.P. J. Org. Chem. 2004, 69, 7359.
395. Liu, M.T.H. Chemistry of Diazirines, 2 Vols, CRC Press, Boca Raton, FL, 1987. For reviews, see Moss, R.A. Acc. Chem. Res. 2006, 39, 267; Liu, M.T.H. Chem. Soc. Rev. 1982, 11, 127.
396. Moss, R.A.; Fu, X. Org. Lett. 2004, 6, 3353.
397. Fede, J.-M.; Jockusch, S.; Lin, N.; Moss, R.A.; Turro, N.J. Org. Lett. 2003, 5, 5027.
398. Toscano, J.P.; Platz, M.S.; Nikolaev, V.; Cao, Y.; Zimmt, M.B. J. Am. Chem. Soc. 1996, 118, 3527.
399. For a review, see Nefedov, O.M.; D'yachenko, A.I.; Prokof'ev, A.K. Russ. Chem. Rev. 1977, 46, 941.
400. For a discussion of the nucleophilcity of dichlorocarbene, see Moss, R.A.; Zhang, M.; Krogh-Jespersen, K. Org. Lett. 2009, 11, 1947.
401. Tomioka, H.; Ozaki, Y.; Izawa, Y. Tetrahedron 1985, 41, 4987.
402. Krogh-Jespersen, K.; Yan, S.; Moss, R.A. J. Am. Chem. Soc. 1999, 121, 6269.
403. Ruck, R. T.; Jones, Jr., M. Tetrahedron Lett. 1998, 39, 2277.
404. Khan, M. I.; Goodman, J. L. J. Am. Chem. Soc. 1995, 117, 6635.
405. Sun, Y.; Tippmann, E. M.; Platz, M. S. Org. Lett. 2003, 5, 1305.
406. Ruck, R.T.; Jones, Jr., M. Tetrahedron Lett. 1998, 39, 2277.
407. See Halberstadt, M.L.; McNesby, J.R. J. Am. Chem. Soc. 1967, 89, 3417.
408. See Buncel, E.; Wilson, H. J. Chem. Educ. 1987, 64, 475; Johnson, C.D. Tetrahedron 1980, 36, 3461; Chem. Rev. 1975, 75, 755; Giese, B. Angew. Chem. Int. Ed. 1977, 16, 125; Pross, A. Adv. Phys. Org. Chem. 1977, 14, 69. See also, Srinivasan, C.; Shunmugasundaram, A.; Arumugam, N. J. Chem. Soc. Perkin Trans. 2 1985, 17; Bordwell, F.G.; Branca, J.C.; Cripe, T.A. Isr. J. Chem. 1985, 26, 357; Formosinho, S.J. J. Chem. Soc. Perkin Trans. 2 1988, 839; Johnson, C.D.; Stratton, B. J. Chem. Soc. Perkin Trans. 2 1988, 1903. For a group of papers on this subject, see Isr. J. Chem. 1985, 26, 303.
409. Closs, G.L.; Coyle, J.J. J. Am. Chem. Soc. 1965, 87, 4270.
410. See Tomioka, H.; Ozaki, Y.; Izawa, Y. Tetrahedron 1985, 41, 4987; Frey, H.M.; Walsh, R.; Watts, I.M. J. Chem. Soc. Chem. Commun. 1989, 284.
411. For a discussion, see Regitz, M. Angew. Chem. Int. Ed. 1991, 30, 674.
412. Arduengo, III, A.J.; Harlow, R.L.; Kline, M. J. Am. Chem. Soc. 1991, 113, 361.
413. See Locatelli, F.; Candy, J.-P.; Didillon, B.; Niccolai, G.P.; Uzio, D.; Basset, J.-M. J. Am. Chem. Soc. 2001, 123, 1658; Brown, R.F.C. Pyrolytic Methods in Organic Chemistry, Academic Press, NY, 1980, pp. 115–163; Wentrup, C. Adv. Heterocycl. Chem,. 1981, 28, 231; Jones, W.M. in de Mayo, P. Rearrangements in Ground and Excited States, Vol. 1, Academic Press, NY, 1980, pp. 95–160; Schaefer, III, H.F. Acc. Chem. Res. 1979, 12, 288; Kirmse, W. Carbene Chemistry, 2nd ed., Academic Press, NY, 1971, pp. 457–496.
414. The activation energy for the 1,2-hydrogen shift has been estimated at 1.1 kcal mol–1 (4.5 kJ mol–1), an exceedingly low value: Stevens, I.D.R.; Liu, M.T.H.; Soundararajan, N.; Paike, N. Tetrahedron Lett. 1989, 30, 481. Also see, Pezacki, J. P.; Couture, P.; Dunn, J. A.; Warkentin, J.; Wood, P. D.; Lusztyk, J.; Ford, F.; Platz, M. S. J. Org. Chem. 1999, 64, 4456.
415. Bettinger, H.F.; Rienstra-Kiracofe, J.C.; Hoffman, B.C.; Schaefer, III, H.F.; Baldwin, J.E.; Schleyer, P.v.R. Chem. Commun. 1999, 1515.
416. Liu, M.T.H.; Bonneau, R. J. Am. Chem. Soc. 1989, 111, 6873; Jackson, J.E.; Soundararajan, N.; White, W.; Liu, M.T.H.; Bonneau, R.; Platz, M.S. J. Am. Chem. Soc. 1989, 111, 6874; Ho, G.; Krogh-Jespersen, K.; Moss, R.A.; Shen, S.; Sheridan, R.S.; Subramanian, R. J. Am. Chem. Soc. 1989, 111, 6875; LaVilla, J.A.; Goodman, J.L. J. Am. Chem. Soc. 1989, 111, 6877.
417. Friedman, L.; Shechter, H. J. Am. Chem. Soc. 1960, 82, 1002.
418. McMahon, R.J.; Chapman, O.L. J. Am. Chem. Soc. 1987, 109, 683.
419. Friedman, L.; Berger, J.G. J. Am. Chem. Soc. 1961, 83, 492, 500.
420. For a review, see Jones, W.M. Acc. Chem. Res. 1977, 10, 353.
421. Moss, R.A.; Johnson, L.A.; Kacprzynski, M.; Sauers, R.R. J. Org. Chem. 2003, 68, 5114.
422. See Yao, G.; Rempala, P.; Bashore, C.; Sheridan, R.S. Tetrahedron Lett. 1999, 40, 17.
423. Moss, R. A.; Ma, Y.; Sauers, R. R.; Madni, M. J. Org. Chem. 2004, 69, 3628.
424. Mekley, N.; El-Saidi, M.; Warkentin, J. Can. J. Chem. 2000, 78, 356.
425. Vignolle, J.; Catton, X.; Bourissou, D. Chem. Rev. 2009, 109, 3333.
426. Roth, H.D. J. Am. Chem. Soc. 1971, 93, 1527, 4935, Acc. Chem. Res. 1977, 10, 85.
427. See Scriven, E.F.V. Azides and Nitrenes, Academic Press, NY, 1984; Lwowski, W. React. Intermed. (Wiley) 1985, 3, 305; 1981, 2, 315; 1978, 1, 197; Abramovitch, R.A. in McManus, S.P. Organic Reactive Intermediates, Academic Press, NY, 1973, pp. 127–192; Kuznetsov, M.A.; Ioffe, B.V. Russ. Chem. Rev. 1989, 58, 732 (N- and O-nitrenes); Meth-Cohn, O. Acc. Chem. Res. 1987, 20, 18 (oxycarbonylnitrenes); Abramovitch, R.A.; Sutherland, R.G. Fortsch. Chem. Forsch. 1970, 16, 1 (sulfonyl nitrenes); Ioffe, B.V.; Kuznetsov, M.A. Russ. Chem. Rev. 1972, 41, 131 (N-nitrenes).
428. McClelland, R.A. Tetrahedron 1996, 52, 6823.
429. Kemnitz, C.R.; Karney, W.L.; Borden, W.T. J. Am. Chem. Soc. 1998, 120, 3499.
430. Wasserman, E.; Smolinsky, G.; Yager, W.A. J. Am. Chem. Soc. 1964, 86, 3166. See Carrick, P.G.; Brazier, C.R.; Bernath, P.F.; Engelking, P.C. J. Am. Chem. Soc. 1987, 109, 5100.
431. Smolinsky, G.; Wasserman, E.; Yager, W.A. J. Am. Chem. Soc. 1962, 84, 3220. For a review, see Sheridan, R.S. Org. Photochem. 1987, 8, 159, pp. 159–248.
432. See Sigman, M.E.; Autrey, T.; Schuster, G.B. J. Am. Chem. Soc. 1988, 110, 4297.
433. See Singh, P.N.D.; Mandel, S.M.; Robinson, R.M.; Zhu, Z.; Franz, R.; Ault, B.S.; Gudmundsdottir, A.D. J. Org. Chem. 2003, 68, 7951.
434. Sander, W.; Grote, D.; Kossmann, S.; Neese, F. J. Am. Chem. Soc. 2008, 130, 4396.
435. McConaghy, Jr., J.S.; Lwowski, W. J. Am. Chem. Soc. 1967, 89, 2357, 4450; Mishra, A.; Rice, S.N.; Lwowski, W. J. Org. Chem. 1968, 33, 481.
436. See Dyall, L.K. in Patai, S.; Rappoport, Z. The Chemistry of Functional Groups, Supplement D, pt. 1, Wiley, NY, 1983, pp. 287–320; Dürr, H.; Kober, H. Top. Curr. Chem. 1976, 66, 89; L'Abbé, G. Chem. Rev. 1969, 69, 345.
437. See Subbaraj, A.; Subba Rao, O.; Lwowski, W. J. Org. Chem. 1989, 54, 3945.
438. See Abramovitch, R.A.; Kyba, E.P. J. Am. Chem. Soc. 1971, 93, 1537.
439. Maltsev, A.; Bally, T.; Tsao, M.-L.; Platz, M. S.; Kuhn, A.; Vosswinkel, M.; Wentrup, C. J. Am. Chem. Soc. 2004, 126, 237.
440. See, for example, Leyva, E.; Platz, M.S.; Persy, G.; Wirz, J. J. Am. Chem. Soc. 1986, 108, 3783.
441. Novak, M.; Rajagopal, S. Adv. Phys. Org. Chem. 2001, 36, 167; Falvey, D. E. in Moss, R. A.; Platz, M. S.; Jones, Jr., M. Reactve Intermediate Chemistry, Wiley–Interscience, Hoboken, NJ, 2004, Vol. 1, pp 593–650.
442. Winter, A.H.; Falvey, D.E.; Cramer, C.J. J. Am. Chem. Soc., 2004, 126, 9661.
443. See Abramovitch, R.A.; Jeyaraman, R. in Scriven, E.F.V. Azides and Nitrenes, Acaademic Press, NY, 1984, pp. 297–357; Gassman, P.G. Acc. Chem. Res. 1970, 3, 26; Lansbury, P.T. in Lwowski, W. Nitrenes, Wiley, NY, 1970, pp. 405–419.
444. Gassman, P.G.; Cryberg, R.L. J. Am. Chem. Soc. 1969, 91, 5176.