March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 6. Mechanisms and Methods of Determining Them
6.D. Kinetic Requirements For Reaction
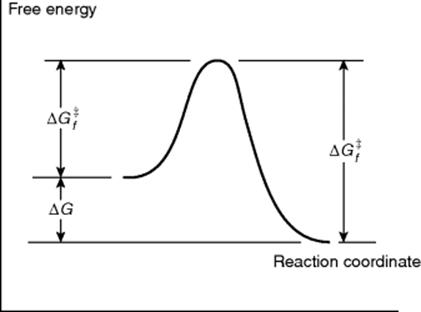
Just because a reaction has a negative ΔG does not necessarily mean that it will take place in a reasonable period of time. A negative ΔG is a necessary, but not a sufficient, condition for a reaction to occur spontaneously. For example, the reaction between H2 and O2 to give H2O has a large negative ΔG, but mixtures of H2 and O2 can be kept at room temperature for many centuries without reacting to any significant extent. In order for a reaction to take place, free energy of activation (ΔG‡) must be added.5 This situation is illustrated in Fig. 6.1,6 which is an energy profile for a one-step reaction without an intermediate. In this type of diagram, the horizontal axis (called the reaction coordinate)7 signifies the progression of the reaction. The parameter ![]() is the free energy of activation for the forward reaction. If the reaction shown in Fig. 6.1 is reversible,
is the free energy of activation for the forward reaction. If the reaction shown in Fig. 6.1 is reversible, ![]() must be >
must be > ![]() , since it is the sum of ΔG and
, since it is the sum of ΔG and ![]() .
.
Fig. 6.1 Free energy profile of a reaction without an intermediate where the products have a lower free energy than the reactants.
When a reaction between two or more molecules has progressed to the point corresponding to the top of the curve, the term transition state is applied to the positions of the nuclei and electrons. The transition state possesses a definite geometry and charge distribution, but has no finite existence; the system passes through it. The system at this point is called an activated complex.8
In transition-state theory,9 the starting materials and the activated complex are taken to be in equilibrium, the equilibrium constant being designated K‡. According to the theory, all activated complexes go on to product at the same rate (which, although at first sight is surprising, is not unreasonable since they are all “falling downhill”) so that the rate constant (see Sec. 6.J.vi) of the reaction depends only on the position of the equilibrium between the starting materials and the activated complex, (i.e., on the value of K‡). The parameter ΔG‡ is related to K‡ by
![]()
so that a higher value of ΔG‡ is associated with a smaller rate constant. The rates of nearly all reactions increase with increasing temperature because the additional energy thus supplied helps the molecules to overcome the activation energy barrier.10 Some reactions have no free energy of activation at all, meaning that K‡ is essentially infinite and that virtually all collisions lead to reaction. Such processes are said to be diffusion controlled.11
Like ΔG, ΔG‡ is made up of enthalpy and entropy components
![]()
ΔH‡, the enthalpy of activation, is the difference in bond energies, including strain, resonance, and solvation energies, between the starting compounds and the transition state. In many reactions, bonds have been broken or partially broken by the time the transition state is reached; the energy necessary for this is ΔH‡. It is true that additional energy will be supplied by the formation of new bonds, but if this occurs after the transition state, it can affect only ΔHand not ΔH‡.
Entropy of activation, (ΔS‡), which is the difference in entropy between the starting compounds and the transition state, becomes important when two reacting molecules must approach each other in a specific orientation in order for the reaction to take place. For example, the reaction between a simple non-cyclic alkyl chloride and hydroxide ion to give an alkene (Reaction 17-13) takes place only if, in the transition state, the reactants are oriented as shown. This is an acid–base reaction because the proton on the carbon β to the chlorine is polarized δ+, and is a weak acid. Removal of that proton initiates loss of the chlorine atom and formation of the alkene. The electrons in the C–H bond (the acidic proton) must align anti to the leaving groups (Cl) for the reaction to proceed.12
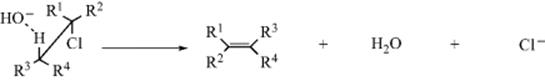
When the two reacting molecules collide, if the ![]() should approach the molecule, the chlorine atom, or near R1 or R2, no reaction can take place. In order for a reaction to occur, the molecules must surrender the freedom they normally have to assume many possible arrangements in space because only one leads to reaction. Thus, a considerable loss in entropy is involved, (i.e., ΔS‡ is negative).
should approach the molecule, the chlorine atom, or near R1 or R2, no reaction can take place. In order for a reaction to occur, the molecules must surrender the freedom they normally have to assume many possible arrangements in space because only one leads to reaction. Thus, a considerable loss in entropy is involved, (i.e., ΔS‡ is negative).
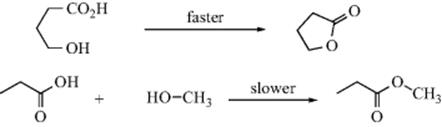
Entropy of activation is also responsible for the difficulty in closing rings13 larger then six membered. Consider a ring-closing reaction in which the two groups that must interact are situated on the ends of a 10-carbon chain. In order for reaction to take place, the groups must encounter each other. But a 10-carbon chain has many conformations, and in only a few of these are the ends of the chain near each other. Thus, forming the transition state requires a great loss of entropy.14 This factor is also present, although less so, in closing rings of six members or less (except three-membered rings), but with rings of this size the entropy loss is less than that of bringing two individual molecules together. For example, a reaction between an OH group and a COOH group in the same molecule to form a lactone with a five- or six-membered ring takes place much faster than the same reaction between a molecule containing an OH group and another containing a COOH group. Although ΔH‡ is about the same, ΔS‡ is much less for the cyclic case. However, if the ring to be closed has three or four members, small-angle strain is introduced and the favorable ΔS‡ may not be sufficient to overcome the unfavorable ΔH‡ change. Table 6.115 shows the relative rate constants for the closing of rings of 3–23 members all by the same reaction.15 Reactions in which the transition state has more disorder than the starting compounds, for example, the pyrolytic conversion of cyclopropane to propene, have positive ΔS‡ values and are thus favored by the entropy effect.
Table 6.115Relative Rate Constants at 50 °Ca
![]()
Ring Size
Relative Rate
3
21.7
4
5.4 × 103
5
1.5 × 106
6
1.7 × 104
7
97.3
8
1.00
9
1.12
10
3.35
11
8.51
12
10.6
13
32.2
14
41.9
15
45.1
16
52.0
18
51.2
23
60.4
a. The rate for an eight-membered ring = 1 for the reaction.
Reprinted with permission from Mandolini, L. J. Am. Chem. Soc. 1978, 100, 550. Copyright © 1978 American Chemical Society. Reprinted with permission from Galli, C.; Illuminati, G.; Mandolini, L., Tamborra, P. J. Am. Chem. Soc. 1977, 99, 2591. Copyright © 1977 American Chemical Society.
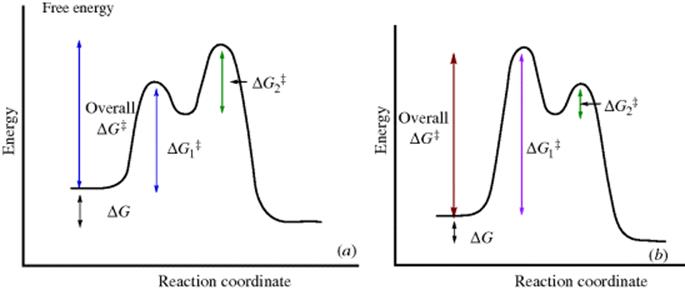
Reactions with intermediates are two-step (or more) processes. In these reactions, there is an energy “well”. There are two transition states, each with an energy higher than the intermediate (Fig. 6.2). The deeper the well, the more stable the intermediate. In Fig. 6.2a, the second peak is higher than the first. The opposite situation is shown in Fig. 6.2b. Note that in reactions in which the second peak is higher than the first, the overall ΔG‡ is less than the sum of the ΔG‡ values for the two steps. Minima in free energy profile diagrams (intermediates) correspond to real species that have a finite though usually short existence. These may be the carbocations, carbanions, free radicals, and so on., discussed in Chapter 5 or molecules in which all the atoms have their normal valences. In either case, under the reaction conditions they do not live long (because ![]() is small), but rapidly go on to products. Maxima in these curves, however, do not correspond to actual species, but only to transition states in which bond breaking and/or bond making have partially taken place. Transition states have only a transient existence with an essentially zero lifetime.16
is small), but rapidly go on to products. Maxima in these curves, however, do not correspond to actual species, but only to transition states in which bond breaking and/or bond making have partially taken place. Transition states have only a transient existence with an essentially zero lifetime.16
Fig. 6.2 (a) Free energy profile for a reaction with an intermediate ![]() and
and ![]() are the free energy of activation for the first and second stages, respectively. (b) Free energy profile for a reaction with an intermediate in which the first peak is higher than the second
are the free energy of activation for the first and second stages, respectively. (b) Free energy profile for a reaction with an intermediate in which the first peak is higher than the second