March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 1. Localized Chemical Bonding
1.E. Photoelectron Spectroscopy
Based on the hybridization model, methane is expected to have four equivalent σ bonds. Indeed, the four bonds of methane are equivalent according to most physical and chemical methods of detection. The nuclear magnetic resonance (NMR) and the infrared (IR) spectrum of methane show no peaks that can be attributed to different kinds of C–H bonds. However, there is one physical technique showing that the eight valence electrons of methane can be differentiated. In this technique, called photoelectron spectroscopy (PES),16 a molecule or free atom is bombarded with vacuum ultraviolet (UV) radiation, causing an electron to be ejected. The energy of the ejected electron can be measured, and the difference between the energy of the radiation used and that of the ejected electron is the ionization potential of that electron. A molecule that contains several electrons of differing energies can lose any one of them as long as its ionization potential is less than the energy of the radiation used. A single molecule loses only one electron; the loss of two electrons by any individual molecule almost never occurs. Since electrons reside in orbitals, a photoelectron spectrum consists of a series of bands, each corresponding to an orbital of a different energy. The spectrum gives a direct experimental picture of all orbitals that are present, and they are ejected in ascending order of their energies, provided that radiation of sufficiently high energy is used.17 Broad bands usually correspond to strongly bonding electrons and narrow bands to weakly bonding or nonbonding electrons.
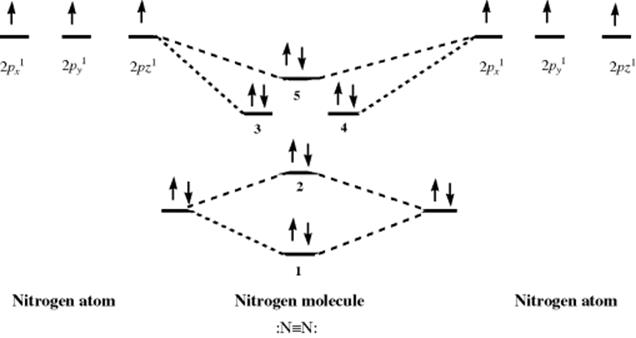
Using PES, it is possible to probe the validity of the hybridization model for bonding. Dinitrogen (N2) is a typical diatomic molecule and is shown in Fig. 1.8.18 The N2 molecule has the electronic structure shown in Fig. 1.9: The two 2s orbitals of the nitrogen atoms combine to give the two orbitals marked 1 (bonding) and 2 (antibonding), while the six 2p orbitals combine to give six orbitals, three of which (marked 3, 4, and 5) are bonding. The three antibonding orbitals (not indicated in Fig. 1.9) are unoccupied. Electrons ejected from orbital 1 are not found in Fig. 1.8 because the ionization potential of these electrons is greater than the energy of the light used (they can be seen when higher energy light is used). The broad band in Fig. 1.8 corresponds to the four electrons in the degenerate orbitals 3 and 4. The individual peaks within this band are caused by different vibrational levels (see Chap. 7). The triple bond of N2 is therefore composed of these two orbitals and orbital 1. The bands corresponding to orbitals 2 and 5 are narrow; hence these orbitals contribute little to the bonding and may be regarded as the two unshared pairs of ![]() . Note that this result is contrary to that expected from a naive consideration of orbital overlaps, where it would be expected that the two unshared pairs would be those of orbitals 1 and 2, resulting from the overlap of the filled 2s orbitals. In addition, the triple bond would be composed of orbitals 3, 4, and 5, resulting from overlap of the p orbitals. This example is one illustration of the value of PES.
. Note that this result is contrary to that expected from a naive consideration of orbital overlaps, where it would be expected that the two unshared pairs would be those of orbitals 1 and 2, resulting from the overlap of the filled 2s orbitals. In addition, the triple bond would be composed of orbitals 3, 4, and 5, resulting from overlap of the p orbitals. This example is one illustration of the value of PES.
Fig. 1.8 Photoelectron spectrum of N2.18 [Reprinted with permission from Brundle, C.R.; Robin, M.B. in Nachod, F.C.; Zuckerman, J.J. Determination of Organic Structures by Physical Methods, Vol. 1, Academic Press, NY, 1971, p. 18. Copyright © 1971, with permission from Elsevier Science. With permission of C. Richard Brundle, 2012.]
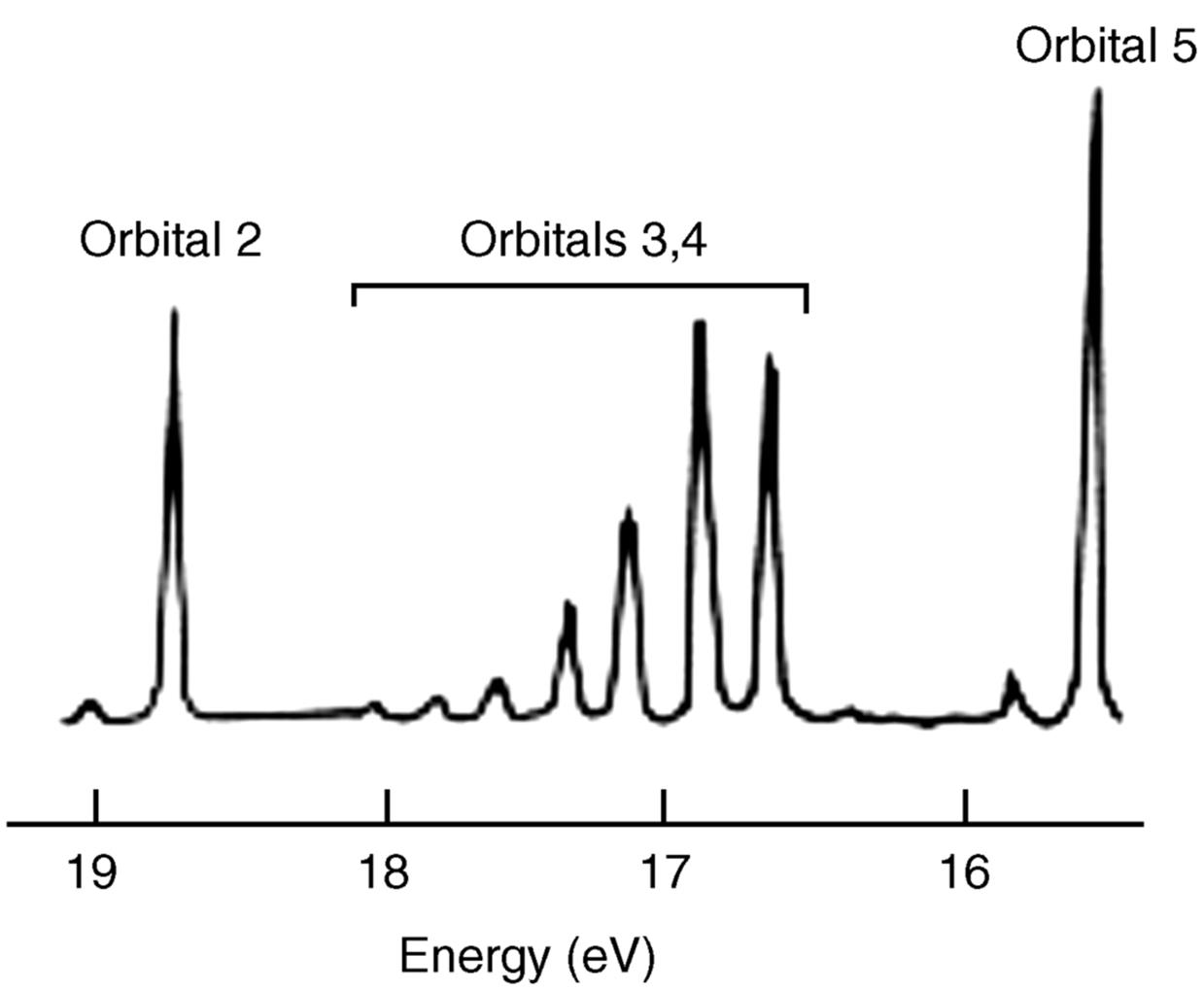
Fig. 1.9 Electronic structure of N2 (inner-shell electrons omitted).
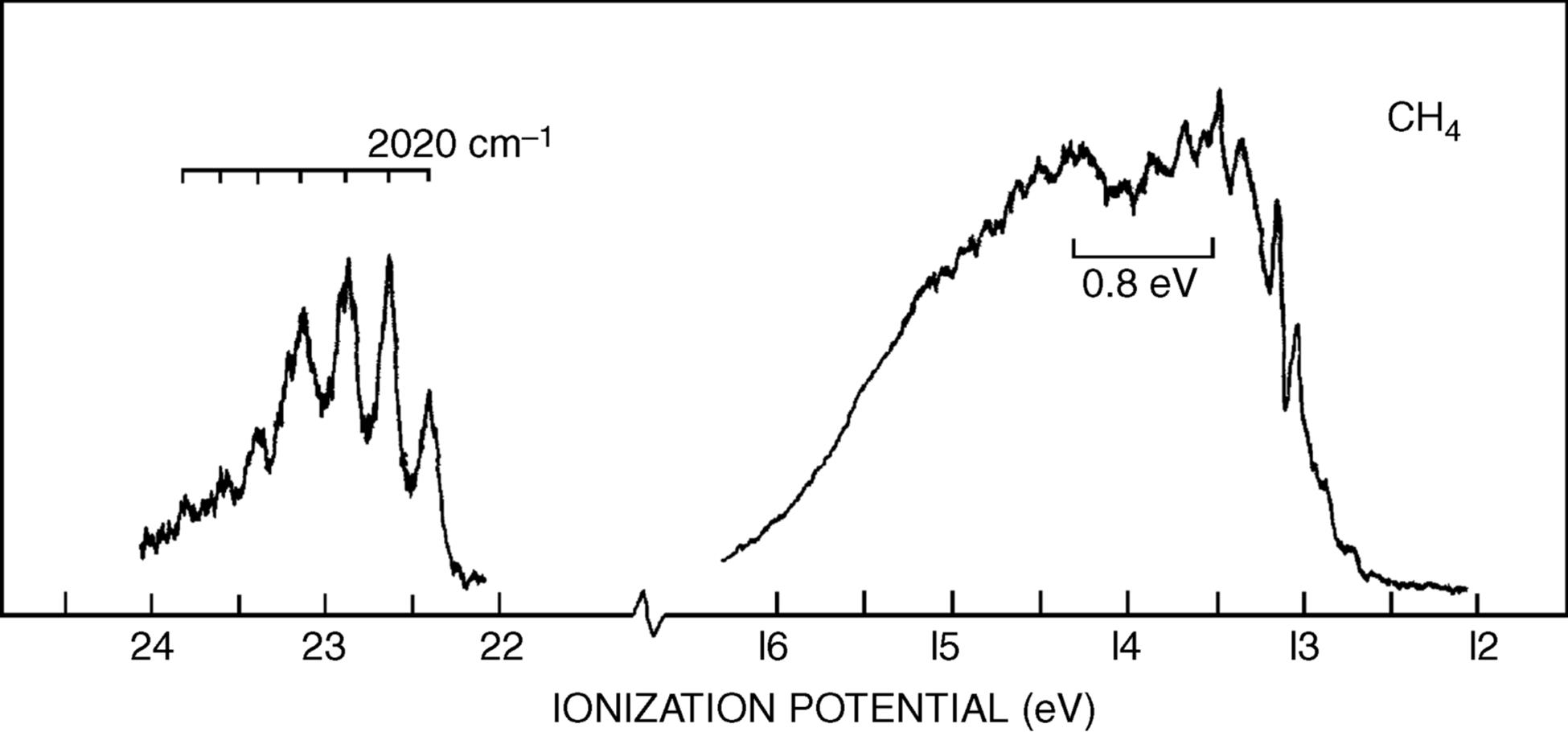
The photoelectron spectrum of methane19 in Fig. 1.10 shows two bands,20 at ~23 and 14 eV, and not the single band expected from the equivalency of the four C–H bonds. Indeed, Fig. 1.10 suggests that carbon uses the available orbitals to form four bonds and the electrons in the bonds are distributed between carbon and the four atoms involved in the bonds. Remember that the hybridization model predicts four identical σ bonds made by overlap of four identical hybrid orbitals. The band at 23 eV comes from two electrons in a low-energy level (called the a1 level), which can be regarded as arising from a combination of the 2s orbital of carbon with an appropriate combination of hydrogen 1s orbitals. The band at 14 eV comes from six electrons in a triply degenerate level (the t2 level), arising from a combination of the three 2p orbitals of carbon with other combinations of 1s hydrogen orbitals. As mentioned above, most physical and chemical processes cannot distinguish these levels, but PES can. The photoelectron spectra of many other organic molecules are known as well,21 including monocyclic alkenes, in which bands < 10 eV are due to π-orbital ionization and those >10 eV originate from ionization of σ orbitals only.22 Note that ordinary sp3 hybridization is not adequate to explain phenomena involving ionized molecules (e.g., the ![]() radical ion, which is left behind when an electron is ejected from methane). For these phenomena, it is necessary to use other combinations of atomic orbitals (see Sec. 1.C).
radical ion, which is left behind when an electron is ejected from methane). For these phenomena, it is necessary to use other combinations of atomic orbitals (see Sec. 1.C).
Fig. 1.10 Photoelectron spectroscopy scan of methane. [Reprinted with permission from Brundle, C.R.; Robin, M.B. J. Chem. Phys. 1970 , 53, 2196. Copyright © 1970, American Institute of Physics.]