March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 9. Effects of Structure and Medium on Reactivity
When the equation for a reaction of, say, carboxylic acids, is written, it is customary to use the formula RCOOH, where R is a generic alkyl group, which implies that all carboxylic acids undergo the reaction. Since most compounds with a given functional group usually give more or less the same reactions, the custom is useful, and the practice is used in this text. It allows a large number of individual reactions to be classified together and serves as an aid both for memory and understanding. Nevertheless, it must be borne in mind that a given functional group does not always react the same way, regardless of what molecule it is a part of. In other words, a reaction at the functional group is influenced by the rest of the molecule. This influence may be great enough to stop the reaction completely or to make it take an entirely different course. Even when two compounds with the same functional group undergo the same reaction, the rates and/or the positions of equilibrium are usually different, sometimes slightly, sometimes greatly, depending on the structures of the compounds. The greatest variations may be expected when additional functional groups are present.
The effects of structure on reactivity can be divided into three major types: field, resonance (or mesomeric), and steric.1 In most cases, two or all three of these are operating, and it is usually not easy to tell how much of the rate enhancement (or decrease) is caused by each of the three effects.
9.A. Resonance and Field Effects
It is often particularly difficult to separate resonance and field effects; they are frequently grouped together under the heading of electrical effects.2 Field effects were discussed in Section 1.I. Table 1.3 contains a list of some +I and −I groups. As for resonance effects, in Section 2.F it was shown how the electron density distribution in aniline is not the same as it would be if there were no resonance interaction between the ring and the NH2 group. Most groups that contain an unshared pair on an atom connected to an unsaturated system display a similar effect; that is, the electron density on the group is less than expected, and the density on the unsaturated system is greater. Such groups are said to be electron donating by the resonance effect (+M groups). Alkyl groups, which do not have an unshared pair, are also +M groups, presumably because of hyperconjugation (see Sec. 2.M).
On the other hand, groups that have a multiple-bonded electronegative atom directly connected to an unsaturated system are −M groups. In such cases, canonical forms can be drawn in which electrons are delocalized from the unsaturated system into the group, as in nitrobenzene (1). Table 9.1 contains a list of some +M and −M groups.
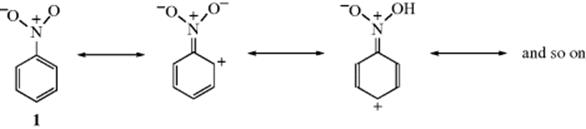
Table 9.1 Some Groups with +M and −M Effects, Not Listed in Order of Strength of Effecta
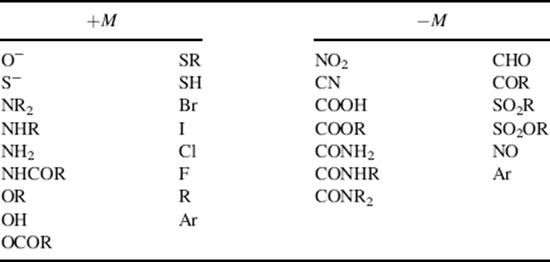
a. Argon (Ar) appears in both lists because it is capable of both kinds of effect.
The resonance effect of a group, whether +M or −M, operates only when the group is directly connected to an unsaturated system, so that, for example, in explaining the effect of the CH3O group on the reactivity of the COOH in CH3OCH2CH2COOH, only the field effect of the CH3O need be considered. This is one way of separating the two effects. In p-methoxybenzoic acid both effects must be considered. The field effect operates through space, solvent molecules, or the σ bonds of a system, while the resonance effect operates through π electrons.
It must be emphasized once again that neither by the resonance nor by the field effect are any electrons actually being donated or withdrawn, though these terms are convenient (and we will use them). As a result of both effects, the electron-density distribution is not the same as it would be without the effect (see Sec. 1.I, 2.F). Complicating the study of these effects on the reactivity of compounds is the fact that a given group may have an effect in the transition state that is considerably more or less than it has in the molecule that does not react.
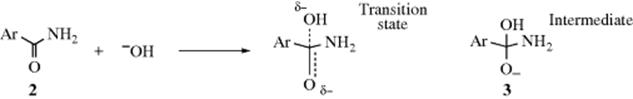
In the alkaline hydrolysis of aromatic amides (Reaction 16-60), the rate-determining step is the attack of hydroxide ion at the carbonyl carbon. The conversion of 2 to 3 illustrates the nature of electrical effects (resonance and field) on reactivity. In the transition state, which has a structure somewhere between that of the starting amide (2) and the intermediate (3), the electron density on the carbonyl carbon is increased. Therefore, electron-withdrawing groups (−I or −M) on the aromatic ring will lower the free energy of the transition state (by spreading the negative charge). These groups have much less effect on the free energy of 2. Since G is lowered for the transition state, but not substantially for 2, ΔG‡ is lowered and the reaction rate is increased (Chapter 6). Conversely, electron-donating groups (+I or +M) should decrease the rate of this reaction. Of course, many groups are −I and +M, and for these it is not always possible to predict which effect will predominate.