March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part II. Introduction
II.B. IUPAC System for Symbolic Representation of Mechanisms
In addition to providing a system for naming transformations, the IUPAC Commission on Physical Organic Chemistry has also produced one for representing mechanisms.4 As will be seen, many mechanisms (but by no means all) are commonly referred to by designations (e.g., SN2, AAC2, E1cB, and SRN1), many of them devised by C.K. Ingold and co-workers. While these designations have been useful (and we will continue to use them in this book), the sheer number of them can be confusing, especially since the symbols do not give a direct clue to what is happening. For example, there is no way to tell directly from the symbols how SN2′ is related to SN2 (see Sec. 10.A.i). The IUPAC system is based on a very simple description of bond changes.5 The letter A represents formation of a bond (association); D the breaking of a bond (dissociation). These are primitive changes. The basic description of a mechanism consists of these letters, with subscripts to indicate where the electrons are going. In any mechanism, the core atoms are defined as (1) the two atoms in a multiple bond that undergoes addition, or (2) the two atoms that will be in a multiple bond after elimination, or (3) the single atom at which substitution takes place.
As an example of the system, this is how an E1cB mechanism (Sec. 17.A.iii) would be represented:
Step 1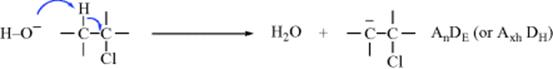
Step 2![]()
Overall designation: AnDE + DN (or AxhDH + DN). In this case, the overall reaction is

and the core atoms are the two carbons in boldface.
Step 1, First Symbol.
A bond is being formed between O and H. Bond formation is represented by A. For this particular case, the system gives two choices for subscript. In any process, the subscript is N if a core atom is forming a bond to a nucleophile (AN) or breaking a bond to a nucleofuge (DN). If a noncore atom is doing the same thing, lowercase n is used instead. Since H and O are noncore atoms, the lowercase n is used, and the formation of the O–H bond is designated by An. However, because involvement of H+ is so common in organic mechanisms, the rules allow an alternative. The subscript H or h may replace N or n. The symbol xh denotes that the H+ comes from or goes to an unspecified carrier atom X. Thus the term Axh means that a bond is being formed between H (moving without electrons) and an outside atom, in this case O. The same subscript, xh, would be used if the outside atom were any other nucleophilic atom, say, N or S.
Step 1,
Second Symbol. A bond is being broken between C and H. The symbol is D. In any process, the subscript is E if a core atom is forming a bond to an electrophile (AE) or breaking a bond to an electrofuge (DE). Since C is a core atom, the symbol here is DE. Alternatively, the symbol could be DH. The rules allow AH or DH to replace AE or DE if the electrophile or electrofuge is H+. Because a core atom is involved in this primitive change the H in the subscript is capitalized.
Step 1. Combined Symbols.
In Step 1, two bond changes take place simultaneously. In such cases, they are written together with no space or punctuation: AnDE or AxhDH
Step 2.
Only one bond is broken in this step and no bonds are formed. (The movement of a pair of unshared electrons into the C–C bond, forming a double bond, is not designated by any symbol. In this system, bond multiplicity changes are understood without being specified.) Thus the symbol is D. The broken bond is between a core atom (C) and a nucleofuge (Cl), so the designation is DN.
The overall designation can be either AnDN + DN or AxhDH + DN. The + symbol shows that there are two separate steps. If desired, rate-limiting steps can be shown by the symbol. In this case, if the first step is the slow step [old designation (E1cB)I], the designation would be AnDE + DN or AxhDH + DN.
For most mechanisms (other than rearrangements), there will be only two A or D terms with uppercase subscripts, and the nature of the reaction can be immediately recognized by looking at them. If both are A, the reaction is an addition; if both are D (as in AnDE + DN) it is an elimination. If one is A and the other D, the reaction is a substitution.
Here, we have given only a brief description of the system. Other IUPAC designations will be shown in Part II, where appropriate. For more details, further examples, and additional symbols, see Ref. 4.