March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part II. Introduction
Chapter 10. Aliphatic Substitution, Nucleophilic and Organometallic
10.D. The SNI Mechanism
In a few reactions, nucleophilic substitution proceeds with retention of configuration, even where there is no possibility of a neighboring-group effect. In the SNi mechanism (substitution nucleophilic internal), part of the leaving group must be able to attack the substrate, detaching itself from the rest of the leaving group in the process. The IUPAC designation is DN + ANDe. The first step is the same as the very first step of the SN1 mechanism: dissociation into an intimate ion pair.223 But in the second step part of the leaving group attacks, necessarily from the front since it is unable to get to the rear, which results in retention of configuration.
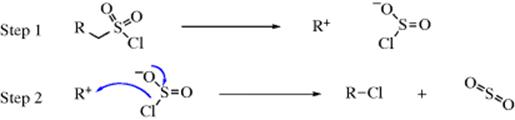
The example shown is the most important case of this mechanism yet discovered, since the reaction of alcohols with thionyl chloride to give alkyl halides usually proceeds in this way, with the first step in this case being ROH + SOCl2 → ROSOCl (these alkyl chlorosulfites can be isolated).
Evidence for this mechanism is as follows: The addition of pyridine to the mixture of alcohol and thionyl chloride results in the formation of alkyl halide with inverted configuration. Inversion results because the pyridine reacts with ROSOCl to give ROSONC5H5 before anything further can take place. The Cl− freed in this process now attacks from the rear. The reaction between alcohols and thionyl chloride is second order, which is predicted by this mechanism, but the decomposition by simple heating of ROSOCl is first order.224
The SNi mechanism is relatively rare. Another example is the decomposition of ROCOCl (alkyl chloroformates) into RCl and CO2.225