March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part II. Introduction
Chapter 10. Aliphatic Substitution, Nucleophilic and Organometallic
10.F. Nucleophilic Substitution at an Aliphatic Trigonal Carbon: The Tetrahedral Mechanism
All the mechanisms so far discussed take place at a saturated carbon atom. Nucleophilic substitution is also important at trigonal carbons, especially when the carbon is double bonded to an oxygen, a sulfur, or a nitrogen. These reactions are discussed in Chapter 16. Nucleophilic substitution at vinylic carbons is considered in Section 10.6 and at aromatic carbons in Chapter 13.
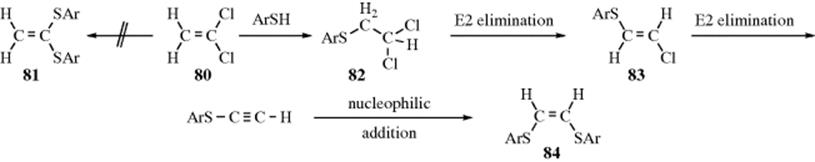
Nucleophilic substitution at a vinylic carbon252 is difficult (see Sec. 10.G.i), but many examples are known. The most common mechanisms are the tetrahedral mechanism and the closely related addition–elimination mechanism. Both of these mechanisms are impossible at a saturated substrate. The addition–elimination mechanism has been demonstrated for the reaction between 1,1-dichloroethene (80) and ArS− catalyzed by −OEt.253 The product was not the 1,1-dithiophenoxy compound (81), but the “rearranged” compound 84. Isolation of 82 and 83 showed that an addition-elimination mechanism had taken place. In the first step, ArSH adds to the double bond (nucleophilic addition, Sec. 15.A.ii) to give the saturated 82. The second step is an E2 elimination reaction (Sec. 17.A.i) to give the alkene 83. A second elimination and addition give 84.
The tetrahedral mechanism, often also called addition–elimination (AdN-E), takes place with much less facility than with carbonyl groups, since the negative charge of the intermediate must be borne by a carbon, which is less electronegative than oxygen, sulfur, or nitrogen:
![]()
Such an intermediate can also stabilize itself by combining with a positive species. When it does, the reaction is nucleophilic addition to a C=C double bond (see Chap 15). It is not surprising that with vinylic substrates addition and substitution often compete. For chloroquinones, where the charge is spread by resonance, tetrahedral intermediates have been isolated:254
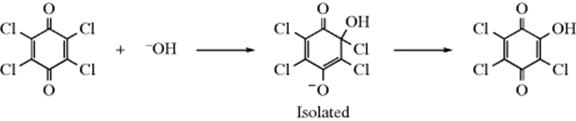
In the case of Ph(MeO)C=C(NO2)Ph + RS−, the intermediate lived long enough to be detected by UV spectroscopy.255
Since both the tetrahedral and addition–elimination mechanisms begin the same way, it is usually difficult to tell them apart, and often no attempt is made to do so. The strongest kind of evidence for the addition–elimination sequence is the occurrence of a “rearrangement”, but of course the mechanism could still take place even if no rearrangement is found. Evidence256 that a tetrahedral or an addition–elimination mechanism takes place in certain cases (as opposed, e.g., to an SN1 or SN2 mechanism) is that the reaction rate increases when the leaving group is changed from Br to Cl to F (this is called the element effect).257 This finding clearly demonstrates that the carbon–halogen bond does not break in the rate-determining step (as it would in both the SN1 and SN2 mechanisms), because fluorine is by far the poorest leaving group among the halogens in both the SN1 and SN2 reactions (Sec. 10.G.iii). The rate is faster with fluorides in the cases cited, because the superior electron-withdrawing character of the fluorine makes the carbon of the C–F bond more positive, and hence more susceptible to nucleophilic attack.
Ordinary vinylic substrates react very poorly if at all by these mechanisms, but substitution is greatly enhanced in substrates of the type ZCH=CHX, where Z is an electron-withdrawing group (HCO, RCO,258 EtOOC, ArSO2, NC, F, etc.), since these β groups stabilize the carbanion:
![]()
Many such examples are known. In most cases, where the stereochemistry has been investigated, retention of configuration is observed,259 but stereoconvergence [the same product mixture from an (E) or (Z) substrate] has also been observed,260 especially where the carbanionic carbon bears two electron-withdrawing groups. Although rare, nucleophilic substitution with inversion has also been reported as in the intramolecular substitution of the C–Br bond of 2-bromobut-2-enylamines by the pendant nitrogen atom, giving 2-ethylene aziridines by way of stereochemical inversion.261 It is not immediately apparent why the tetrahedral mechanism should lead to retention, but this behavior has been ascribed, on the basis of MO calculations, to hyperconjugation involving the carbanionic electron pair and the substituents on the adjacent carbon.262
Vinylic substrates are in general very reluctant to undergo SN1 reactions, but they can be made to do so in two ways:263 (1) By the use of a group that stabilizes the vinylic cation. For example, α-aryl vinylic halides ArBrC=CR′2have often been shown to give SN1 reactions.264 The SN1 reactions have also been demonstrated with other stabilizing groups: cyclopropyl,265 vinylic,266 alkynyl,267 and an adjacent double-bond (R2C=C=CR′X).268 (2) Even without a stabilization, by the use of a very good leaving group [e.g., OSO2CF3 (triflate)].269 The stereochemical outcome of SN1 reactions at a vinylic substrate is often randomization,270 that is, either a cis or a trans substrate gives a 1:1 mixture of cis and trans products, indicating that vinylic cations are linear. Another indication that vinylic cations prefer to be linear is the fact that reactivity in cycloalkenyl systems decreases with decreasing ring size.271 However, a linear vinylic cation need not give random products.272 The empty p orbital lies in the plane of the double bond, so entry of the nucleophile can be and often is influenced by the relative size of R1 and R2.273 It must be emphasized that even where vinylic substrates do give SN1 reactions, the rates are generally lower than those of the corresponding saturated compounds.
Alkynyl cations are so unstable that they cannot be generated even with very good leaving groups. However, one way in which they have been generated was by formation of a tritiated substrate.
![]()
When the tritium (half-life 12.26 years) decays it is converted to the helium-3 isotope, which, of course, does not form covalent bonds, and so immediately departs, leaving behind the alkynyl cation. When this was done in the presence of benzene, RC![]() CC6H5 was isolated.274 The tritium-decay technique has also been used to generate vinylic and aryl cations.275
CC6H5 was isolated.274 The tritium-decay technique has also been used to generate vinylic and aryl cations.275
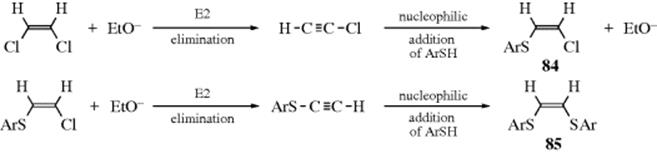
Besides the mechanisms already discussed, another mechanism, involving an elimination–addition sequence, has been observed in vinylic systems (a similar mechanism is known for aromatic substrates, Sec. 13.A.iii). An example of a reaction involving this mechanism is the reaction of 1,2-dichloroethane with ArS− and −OEt to produce 84. The mechanism may be formulated as shown. The steps are the same as in the addition–elimination mechanism, but in reverse order. Evidence for this sequence276 is as follows: (1) The reaction does not proceed without ethoxide ion, and the rate is dependent on the concentration of this ion and not on that of ArS−. (2) Under the same reaction conditions, chloroacetylene gave 84 and then 85. (3) Compound 84, treated with ArS−, gave no reaction but, when EtO− was added, 85 was obtained. It is interesting that the elimination–addition mechanism has even been shown to occur in five- and six-membered cyclic systems, where triple bonds are greatly strained.277 Note that both the addition–elimination and elimination–addition sequences, as shown above, lead to overall retention of configuration, since in each case both addition and elimination are anti.
The elimination–addition sequence has also been demonstrated for certain reactions of saturated substrates (e.g., ArSO2CH2CH2SO2Ar).278 Treatment of this with ethoxide proceeds as follows:
![]()
Mannich bases (see Reaction 16-19) of the type RCOCH2CH2NR2 similarly undergo nucleophilic substitution by the elimination–addition mechanism.279 The nucleophile replaces the NR2 group.
The simple SN2 mechanism has never been convincingly demonstrated for vinylic substrates.280
Vinylic halides can react by a SRN1 mechanism (Sec. 13.A.iv) in some cases. An example is the FeCl2 catalyzed reaction of 1-bromo-2-phenylethene and the enolate anion of pinacolone (t-BuCOCH2−), which gave a low yield of substitution products along with alkynes.281