Organic Chemistry I For Dummies, 2nd Edition (2014)
Part III. Functional Groups
IN THIS PART …
Recognize different functional groups.
See reactions to make alkyl halides and alcohols.
Find out what makes a ring system aromatic.
Understand the reactions of alkyl halides, alcohols, and aromatics.
Chapter 12. Replacing and Removing: Substitution and Elimination Reactions
IN THIS CHAPTER
Seeing second-order substitution
Considering nucleophiles and leaving groups
Seeing first-order substitution
Discovering first-order and second-order elimination reactions
Distinguishing substitution and elimination reactions
Although you see countless reactions in organic chemistry, you usually don’t sweat the details too much, details like what kinds of solvents are ideal for that reaction, or typical side pathways that lead to undesirable byproducts. The substitution and elimination reactions are exceptions, because these are some of the most widely applicable and versatile reactions that you see in organic chemistry. As such, they deserve a closer look. In this chapter, I present the substitution and elimination reactions, and show you how to recognize these types of reactions. With just these two reaction types, you can synthesize more organic molecules than you can shake a stick at.
Group Swap: Substitution Reactions
Substitution reactions follow the form shown in Figure 12-1. The overall reaction is fairly simple — one group simply substitutes for another in the reaction.
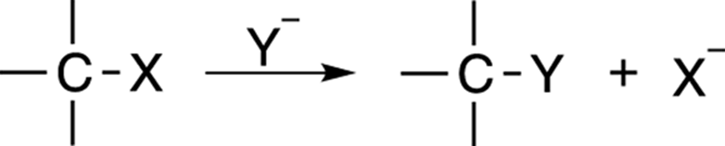
FIGURE 12-1: A substitution reaction.
Two mechanisms for substitution are possible, the SN1 mechanism, and the SN2 mechanism, and they’re shown in Figure 12-2. Both involve substituting one group on a molecule for another. If you think of these substitution reactions in relationship terms, the SN2 mechanism is analogous to dumping your current significant other and immediately starting a relationship with a new romantic partner. The SN1 mechanism is analogous to breaking up with your current significant other, staying single for a while, and only after you’ve been single, becoming attached to a new romantic partner.
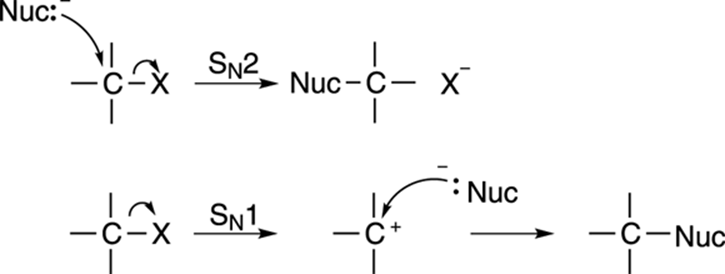
FIGURE 12-2: Two different substitution mechanisms.
So how do you know which mechanism will occur for a given substitution reaction? The answer, unfortunately, is, “It depends.” It depends on the solvent, the nature of the substrate, and the substituting group (called the nucleophile, or nucleus lover, sometimes abbreviated Nuc). To figure out which mechanism will occur, you need to see the details of each mechanism.
Seeing Second-Order Substitution: The SN2 Mechanism
The mechanism for the SN2 reaction is shown in Figure 12-3. This reaction is called the substitution nucleophilic bimolecular reaction, which, thankfully, is called SN2 for short. The SN2 reaction occurs in a single step: A nucleophile (Lewis base) attacks a carbon that’s attached to an electronegative leaving group (labeled X), and gives the leaving group the boot, taking its place.
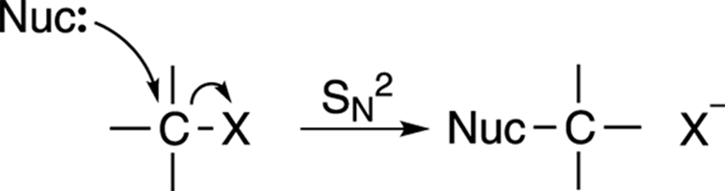
FIGURE 12-3: The SN2 mechanism.
Why does the nucleophile attack the carbon? One way to think of this reaction is in terms of the attraction between opposite charges. The carbon-leaving group bond (C-X) is polarized; that is, the electronegative leaving group pulls electron density away from the carbon to which it’s attached, leaving the carbon with a partially positive charge, as shown in Figure 12-4. (See Chapter 2 for more on electronegativity.) Therefore, the molecule that contains the leaving group (called the substrate) acts as an electrophile (an electron lover). Nucleophiles (electron-rich species that love nuclei) attack the partially positive carbon, in part due to the electrostatic attraction between the two nuclei.
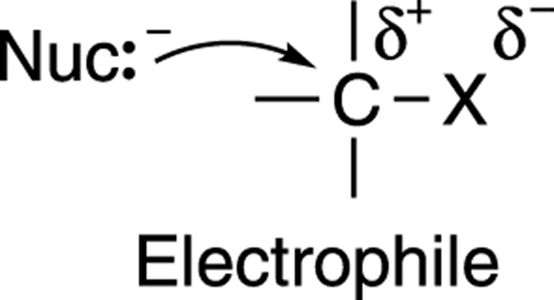
FIGURE 12-4: Nucleophile-electrophile attraction.
 If you understand nucleophile-electrophile attraction in this way, you can understand many, many organic reactions. The basic templates for these reactions are the same: Some electron-rich atom (a nucleophile) attacks an electron-poor atom (an electrophile). The details change, but that’s pretty much the gist of it.
If you understand nucleophile-electrophile attraction in this way, you can understand many, many organic reactions. The basic templates for these reactions are the same: Some electron-rich atom (a nucleophile) attacks an electron-poor atom (an electrophile). The details change, but that’s pretty much the gist of it.
How fast? The rate equation for the SN2 reaction
The rate of the SN2 reaction follows the rate equation: rate = k[substrate][nucleophile]. Looking at this rate equation, you see that the concentration of both the substrate and the nucleophile determine the rate of the SN2 reaction, making the SN2 reaction second order (thus, the “2” in SN2).
The reaction diagram for the SN2 mechanism is shown in Figure 12-5. A reaction diagram displays the energy (labeled E) as a function of time. Every reaction has at least one energy “hill” that must be climbed to transform the starting materials into the products; this “hill” is a barrier called the activation energy, or Ea. The activation barrier is the minimum amount of energy that needs to be supplied to transform the starting material into product.
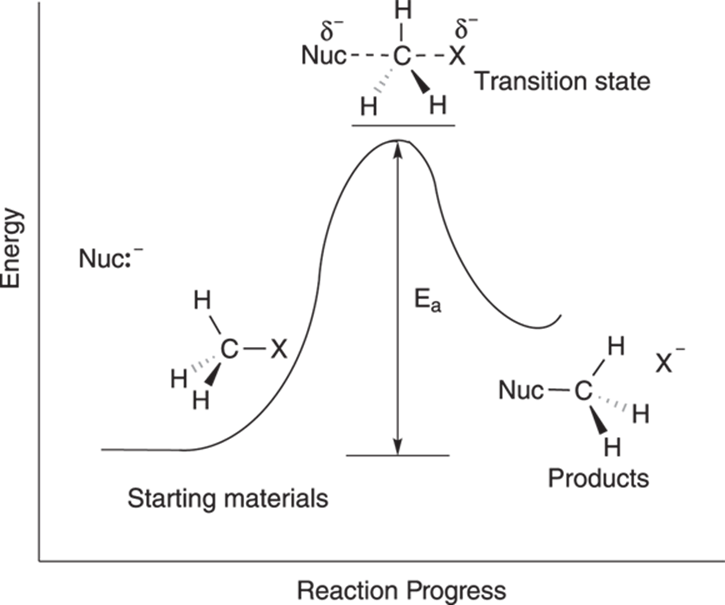
FIGURE 12-5: The SN2 reaction diagram.
The size of the activation barrier determines the rate of the reaction. If the reaction only has to climb a very small energy hill, the reaction will proceed quickly. If the energy hill is high, the reaction will occur much more slowly, just as an athlete runs faster over a small hill than over a mountain.
 The top of the energy hill is the transition state of the reaction. This is the point at which the starting materials are midway through the reaction, when the reactants are at their highest energy level, and when the bonds in the reactants are partially broken and bonds in the products partially formed. A transition state cannot be isolated; it’s not a long-lived intermediate, but simply a transition between the starting materials and products. It’s just a point on the road to transforming one material into the next. A reaction passes through the transition state faster than a chemistry professor can mark an X on an exam.
The top of the energy hill is the transition state of the reaction. This is the point at which the starting materials are midway through the reaction, when the reactants are at their highest energy level, and when the bonds in the reactants are partially broken and bonds in the products partially formed. A transition state cannot be isolated; it’s not a long-lived intermediate, but simply a transition between the starting materials and products. It’s just a point on the road to transforming one material into the next. A reaction passes through the transition state faster than a chemistry professor can mark an X on an exam.
Effect of the substrate on the SN2 reaction
A nucleophile approaching a substrate in order to undergo an SN2 reaction is a lot like a fan approaching a celebrity to get an autograph. If a celebrity has no bodyguards, the fan can easily approach and ask for the autograph. With one bodyguard (I’ll call these bodyguards “R groups”), the fan has a little more difficulty slipping by the bodyguard, but getting the autograph is still doable. The fan may need to wait until the bodyguard lets his guard down and allows the fan to slip past. With two R group bodyguards, the fan would have an even harder time (or a longer wait) before he or she could slip through to the celebrity. But with three bodyguards blocking all lines of approach to the celebrity, the fan might as well forget about getting that precious autograph.
R groups on the carbon that contains the leaving group act as bodyguards, and hinder the approach of the nucleophile, as shown in Figure 12-6. Therefore, SN2 reactions work best with methyl and primary substrates, when the carbon containing the leaving group has no R group bodyguards attached to it (in the case of a methyl substrate) or just one (in the case of a primary substrate). Reactions with substrates that contain two R group bodyguards (secondary substrates) do proceed, but these reactions take place at a slower rate than reactions with primary substrates. But with tertiary substrates (substrates in which the carbon that contains the leaving group has three R groups attached to it), the SN2 reaction says, “No dice,” and you get no reaction. Why? Everything comes back to the mechanism. In the SN2 mechanism, the nucleophile attacks the backside of the substrate. With three big fat bulky R groups blocking the rear approach, the nucleophile can’t get close enough to attack the carbon. Such blocking by bulky groups is called steric hindrance, using organic-speak. Because of such steric hindrance, you won’t see an SN2 reaction on a tertiary substrate.
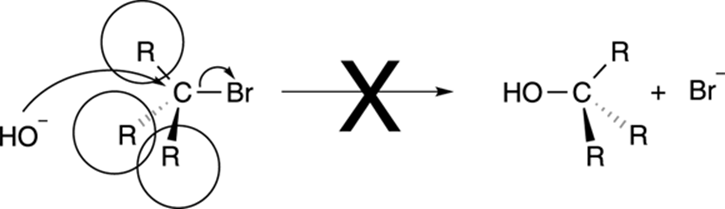
FIGURE 12-6: Steric hindrance can prevent an SN2 reaction.
 SN2 reactions prefer methyl (CH3X) over primary substrates (R-CH2-X), but primary over secondary substrates (R2CHX). SN2 reactions don’t work with tertiary substrates (R3CX).
SN2 reactions prefer methyl (CH3X) over primary substrates (R-CH2-X), but primary over secondary substrates (R2CHX). SN2 reactions don’t work with tertiary substrates (R3CX).
Needs nucleus: The role of the nucleophile
Because the concentration of the nucleophile is included in the rate equation of the SN2 reaction, a good nucleophile is required. That leads to the somewhat sticky question: What makes a good nucleophile? Unfortunately, that question can’t be answered precisely, because the strength of a nucleophile can change by varying the solvent or reaction conditions. Still, some general rules about nucleophile strength can be applied.
 Any molecule with a lone pair of electrons to donate can act as a nucleophile. The strength of a nucleophile (or the nucleophilicity, using organic-speak) generally goes hand-in-hand with basicity. A strong base is usually a strong nucleophile and vice versa. But basicity and nucleophilicity are not the same things. Basicity refers to the ability of a molecule to pluck off a proton, and is defined by the base’s equilibrium constant; nucleophilicity refers to the ability of a lone pair to attack a carbon on an electrophile.
Any molecule with a lone pair of electrons to donate can act as a nucleophile. The strength of a nucleophile (or the nucleophilicity, using organic-speak) generally goes hand-in-hand with basicity. A strong base is usually a strong nucleophile and vice versa. But basicity and nucleophilicity are not the same things. Basicity refers to the ability of a molecule to pluck off a proton, and is defined by the base’s equilibrium constant; nucleophilicity refers to the ability of a lone pair to attack a carbon on an electrophile.
When wouldn’t basicity and nucleophilicity coincide? Usually, these don’t coincide when you have bases/nucleophiles substituted with bulky groups. For example, both methoxide (CH3O–) and t-butoxide ((CH3)3CO–), shown in Figure 12-7, are strong bases, but methoxide is a good nucleophile while t-butoxide is not. The reason for this difference is that t-butoxide has three bulky methyl groups that hinder approach to the substrate (another example of steric hindrance). Therefore, even though t-butoxide is a strong base, it’s a poor nucleophile.
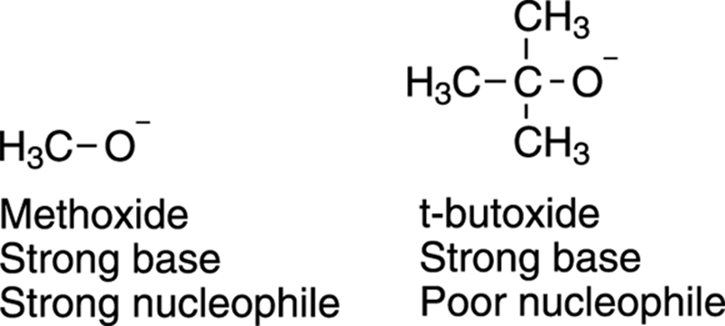
FIGURE 12-7: The nucleophilicity of two strong bases.
 Steric hindrance decreases nucleophilicity.
Steric hindrance decreases nucleophilicity.
In addition to the basicity of a molecule, two other major factors can help you compare the nucleophilicities of molecules:
· Negatively charged nucleophiles are stronger nucleophiles than neutral nucleophiles (just as negatively charged atoms are more basic than neutral atoms). Thus, OH– is a stronger nucleophile than H2O, and HS– is a stronger nucleophile than H2S.
· Typically, nucleophilicity increases as you go down the periodic table. Therefore, H2S is a better nucleophile than H2O because sulfur is one row down on the periodic table from oxygen. Likewise, iodide (I–) is a better nucleophile than bromide (Br–) because iodine is one row down from bromine on the periodic table.
Seeing the SN2 reaction in 3-D: Stereochemistry
In the SN2 reaction, the nucleophile approaches from the back side of the substrate. Therefore, in the product, the three groups on the carbon have inverted, like an umbrella blown inside out by the wind. With the SN2 reaction, then, you get inversion of stereochemistry (see Chapter 6 for more on stereochemistry). For example, with the reaction of 2-bromobutane and hydroxide (see Figure 12-8), the chiral center has an S configuration in the starting material but an R configuration in the product as a result of the inversion of configuration in the SN2 reaction.
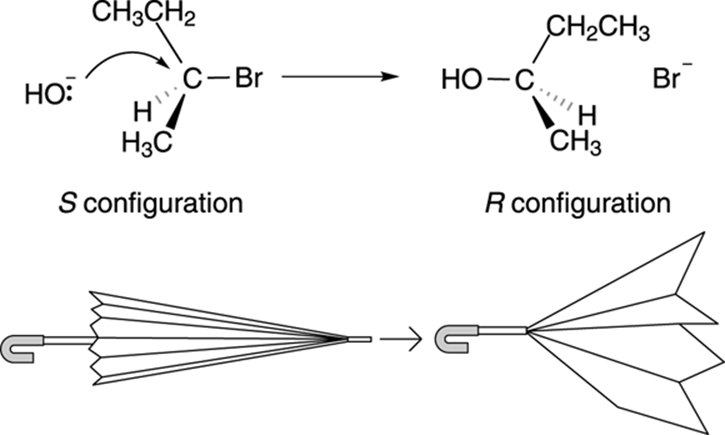
FIGURE 12-8: The SN2 reaction of 2-bromobutane.
 The SN2 reaction leads to an inversion of the stereochemistry.
The SN2 reaction leads to an inversion of the stereochemistry.
Seeing solvent effects
The choice of solvent also affects the SN2 substitution reaction. Not all solvents are created equal; some solvents work better than others in a given reaction. In the SN2 reaction, the preferred solvents are those that are both polar (see Chapter 2) and aprotic. Protic is organic-speak for solvents that contain O-H or N-H bonds; these solvents include alcohols, water, and amines. Aprotic solvents have no N-H or O-H bonds. Good polar aprotic solvents for the SN2 reaction include DMSO (dimethyl sulfoxide), CH2Cl2 (dichloromethane), and ethers (R-O-R).
Why do you need aprotic and not protic solvents for the SN2 reaction? Protic solvents tend to form a solvent “cage” around the nucleophile, as shown for the chloride ion caged by water in Figure 12-9. This cage makes the nucleophile less nucleophilic. A nucleophile in a protic solvent is like a college student surrounded by televisions — it doesn’t feel particularly inclined to go out and do the work that needs to be done. Polar solvents that are aprotic still have the ability to dissolve the polar reactants (because “like dissolves like” as you discovered in your first year of chemistry), but these polar aprotic solvents don’t cage the nucleophile, so they don’t make the nucleophile less nucleophilic.
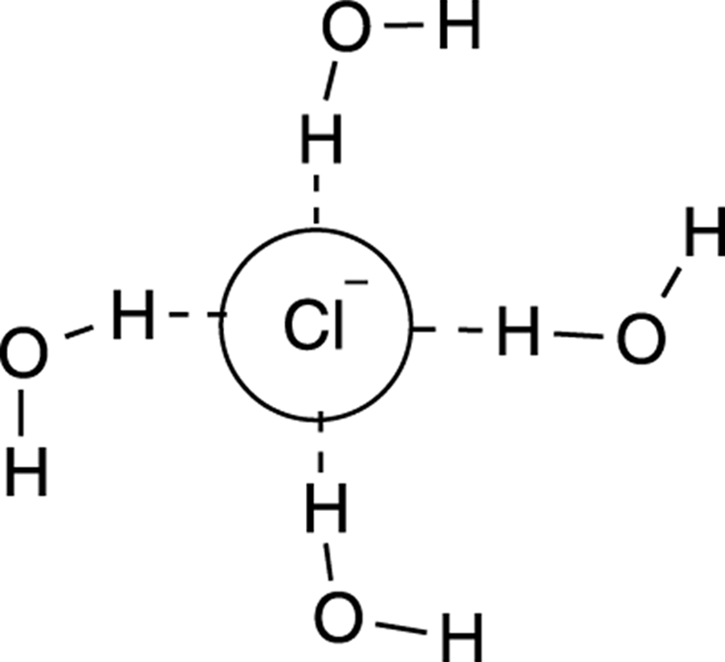
FIGURE 12-9: The solvent cage formed by the protic solvent (water).
 Protic solvents decrease nucleophilicity in SN2 reactions.
Protic solvents decrease nucleophilicity in SN2 reactions.
I’m outta here: The leaving group
Another requirement of the SN2 reaction is that the substrate needs to have a good leaving group. The leaving group is the piece that’s displaced by the nucleophile (often labeled X). Good leaving groups are typically weak bases, and weak bases are the conjugate bases of strong acids (see Chapter 4 for more on acids and bases). Therefore, if you can find a strong acid and deprotonate it, you’ve probably found a good leaving group. The hydrohalic acids (HBr, HCl, and so on) are strong acids, so the halides (like I–, Br–, Cl–) are excellent leaving groups. The halides, in fact, are the most common leaving groups in SN2 reactions. Another good leaving group is tosylate. Tosylate is the conjugate base of the strong acid p-toluenesulfonic acid, and is one of the best leaving groups available (better than all the halides). Figure 12-10 shows the most common leaving groups, and ranks them from best to worst.
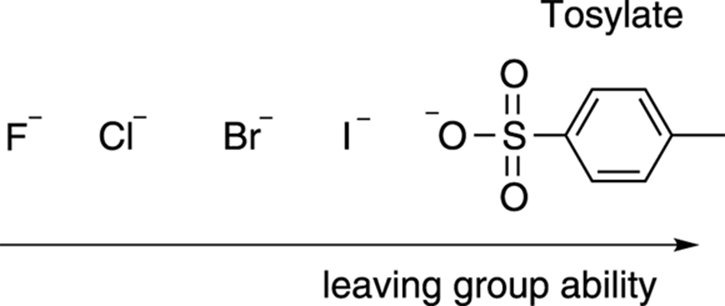
FIGURE 12-10: Leaving groups for the SN2 reaction.
 Hydroxide ion (OH–), alkoxides (RO–), and amide ion (NH2–) are bad leaving groups because they’re strong bases. Show them as leaving groups at your own risk. Because these groups are bad leaving groups, you won’t typically see an SN2 reaction on an ether (which is why you can use ethers as solvents in SN2 reactions), alcohol, alkyl fluoride, or an amine.
Hydroxide ion (OH–), alkoxides (RO–), and amide ion (NH2–) are bad leaving groups because they’re strong bases. Show them as leaving groups at your own risk. Because these groups are bad leaving groups, you won’t typically see an SN2 reaction on an ether (which is why you can use ethers as solvents in SN2 reactions), alcohol, alkyl fluoride, or an amine.
First-Order Substitution: The SN1 Reaction
The other substitution mechanism is the SN1 mechanism, and is shown in Figure 12-11. The SN1 reaction proceeds in two steps. In the first step, the leaving group decides to pack its bags and take off, leaving a carbocation intermediate. Then the nucleophile attacks the carbocation to make the substituted product.
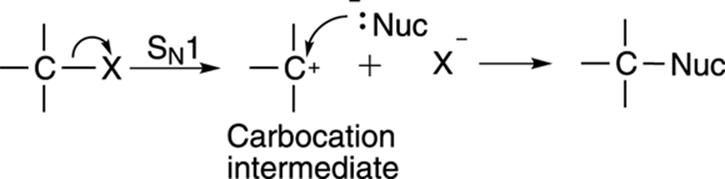
FIGURE 12-11: The SN1 mechanism.
How fast? The rate equation for the SN1 reaction
The rate for the SN1 reaction follows the rate equation: rate = k[substrate].
You may be surprised to see that, unlike the case in the SN2 reaction, the concentration of the nucleophile is not included in the rate equation for the SN1 reaction. The rate equation is first order (thus, the “1” in SN1) and depends only on the concentration of the substrate. Why is the nucleophile not included? You can see why by looking at the reaction diagram, shown in Figure 12-12.
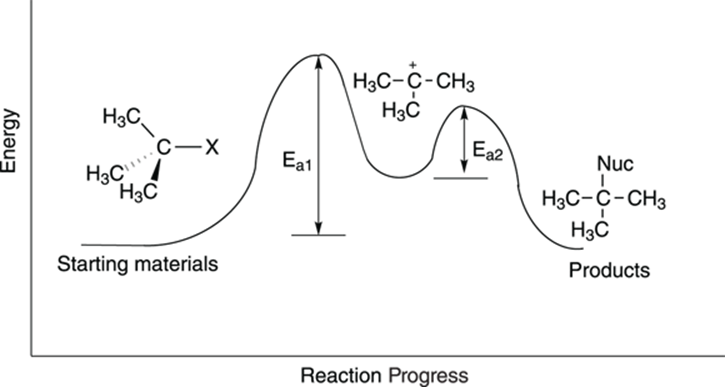
FIGURE 12-12: The SN1 reaction diagram.
From the reaction diagram, you see that two hills need to be climbed to transform the starting materials into the product. The first hill is the taller one, and is the energy barrier for the step in which the leaving group makes like a tree and gets the heck out of there, forming the carbocation. The second step, the attack of the nucleophile on the carbocation, involves a smaller energy hill. Because the first step has the highest activation barrier, the first step is the slowest step, making it the rate-determining step.
You’ve probably heard the phrase “A chain is only as strong as its weakest link.” Similarly, a reaction is only as fast as its slowest step, which is called the rate-determining step. Steps that follow the rate-determining step have no effect on the rate of a reaction. You have a similar situation when you have a tiny washing machine and a huge dryer, and want to know how quickly you can clean your clothes (see Figure 12-13). Is the rate at which you get clean clothes going to increase by increasing the size of your already too-big dryer? No, because the rate determining step is the washing step. Only increasing the size of the washer would increase the rate at which you get clean clothes out of this clothes-cleaning system.
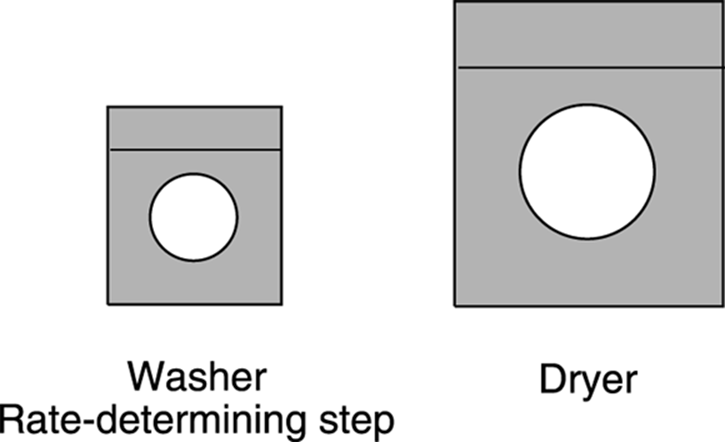
FIGURE 12-13: With this tiny washer, washing is the rate-determining step.
That’s why the nucleophile is not included in the rate equation for the SN1 reaction, because the nucleophile only gets involved in the mechanism after the rate-determining step. Making the nucleophile stronger, or increasing the concentration of the nucleophile, is like making the dryer bigger — it has no effect on the rate of forming product.
Seeing good SN1 substrates
Good substrates for the SN1 reaction are different from those that are good for the SN2 reaction. Good substrates for the SN1 reaction will be substrates that form a stable carbocation upon releasing the leaving group. If you lower the energy of the cation intermediate, you lower the activation energy and, thus, speed up the reaction. So, to find good substrates for the SN1 reaction, you look for those that will lead to a stable intermediate carbocation.
Recall the features that contribute to carbocation stability (refer to Chapter 10 if you need a refresher on carbocations). Carbocations are stabilized by the presence of alkyl groups or by resonance. Thus, tertiary substrates form more stable carbocations than secondary or primary substrates. As a result, tertiary substrates are better SN1 substrates than secondary substrates, which in turn are better SN1 substrates than primary substrates (see Figure 12-14). Substrates with the ability to stabilize the cation through resonance are also good SN1 substrates; these substrates include benzylic and allylic substrates.
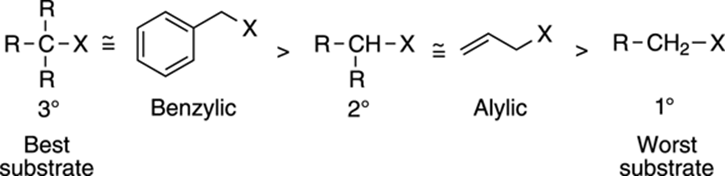
FIGURE 12-14: Substrates for the SN1 reaction.
 Good SN1 substrates are those that can make stable carbocation intermediates.
Good SN1 substrates are those that can make stable carbocation intermediates.
Seeing solvent effects on the SN1 reaction
SN2 reactions prefer aprotic solvents, but SN1 reactions prefer protic solvents. This preference comes from the ability of protic solvents to stabilize the intermediate carbocation (as shown in Figure 12-15), lowering the energy hill for the rate-determining step. Recall that in the SN2 reaction you don’t want protic solvents because they decrease the nucleophile strength. In the SN1 reaction, the strength of the nucleophile has no effect on the rate, so protic solvents (like alcohols and water) are excellent for SN1 reactions. You generally won’t see SN1 reactions in aprotic solvents.

FIGURE 12-15: The stabilizing interaction of a protic solvent (water) with a cation.
 Protic solvents stabilize carbocations in SN1 reactions.
Protic solvents stabilize carbocations in SN1 reactions.
Stereochemistry of the SN1 reaction
Stereochemistry in the SN1 reaction is not as clear-cut as it is in the SN2 reaction. The intermediate in the SN1 reaction is a carbocation, which is sp2 hybridized. As a result, these carbocations are planar, and have an empty p orbital. The empty p orbital could be attacked equally well by the nucleophile’s lone pair from either the top or the bottom of the carbocation, as shown in Figure 12-16. Therefore, you get about a 50/50 mixture of enantiomers from the SN1 reaction, which is called a racemic mixture (see Chapter 6).

FIGURE 12-16: The SN1 reaction of a tertiary alkyl halide.
 SN1 reactions give racemic products.
SN1 reactions give racemic products.
Other fun facts about the SN1 reaction
Because the strength of the nucleophile is unimportant, SN1 reactions work best with weak nucleophiles. However, the SN1 reaction requires a good leaving group (like a halide or tosylate), as the SN2 reaction does. An important detail to remember about the SN1 reaction is the consequence of going through a carbocation intermediate. Recall that carbocations are mischievous little devils, and will rearrange if doing so will lead to a stable cation (see Chapter 10 for more on carbocations). As a result, you occasionally see carbocation rearrangements in the SN1 reaction.
Table 12-1 is a convenient tool for comparing the SN1 and SN2 reaction. If you want to determine whether a substitution reaction will go by the SN1 or the SN2 mechanism, look first at the substrate. If the substrate is primary or methyl, the reaction will most likely proceed by the SN2 mechanism. If the substrate is tertiary, the reaction will proceed by the SN1 mechanism. Secondary substrates are something of a gray area; both reactions will work with these substrates, so you need to look at other features of the reaction (like the solvent) to see which mechanism may be favored over the other. With secondary alkyl substrates, you may also get a mixture of the two mechanisms.
TABLE 12-1 Comparing SN1 and SN2 Reactions
|
SN1 |
SN2 |
|
|
Substrate |
Prefers 3° over 2° |
Prefers 1° over 2° |
|
Rate equation |
rate = k[substrate] |
rate = k[substrate][nucleophile] |
|
Nucleophile |
Nucleophile unimportant |
Requires a good nucleophile |
|
Reaction |
Racemic products |
Inversion of stereochemical configuration |
|
Leaving groups |
Good leaving group required |
Good leaving group required |
|
Solvent |
Prefers polar protic solvents (alcohols, water, and so on) |
Prefers polar aprotic solvents (ethers, halogenated solvents, and so on) |
|
Rearrangements |
Possible |
Not possible |
Seeing Elimination Reactions
Elimination reactions often compete with substitution reactions. The general form of an elimination reaction is shown in Figure 12-17. In this reaction, a substrate (typically, an alkyl halide) eliminates one equivalent (unit) of acid to make an alkene. As in substitution reactions, two possible mechanisms are available for this elimination reaction — the E1 and E2 mechanism — and both elimination reactions have similarities to their substitution counterparts.
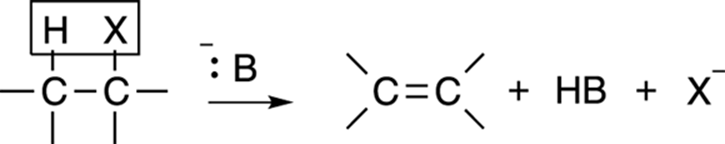
FIGURE 12-17: The elimination reaction.
Seeing second-order eliminations: The E2 reaction
Second-order elimination is called the E2 reaction. Like the SN2 mechanism, the E2 mechanism takes place in a single step, as shown in Figure 12-18. A base plucks off a proton on a carbon adjacent to the leaving group, forming the double bond and giving the leaving group the boot.
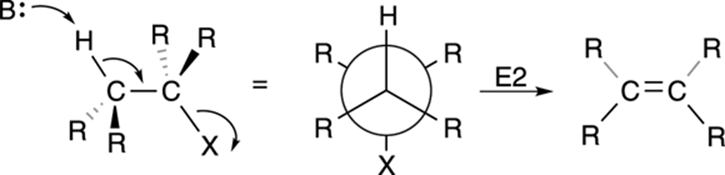
FIGURE 12-18: The E2 elimination mechanism.
One requirement of the E2 reaction is that the hydrogen to be eliminated and the leaving group must typically be in anti-periplanar geometry. To be anti-periplanar means that the hydrogen and the leaving group (as well as the two carbons that will form the double bond) must be on the same plane and on opposite faces of the carbon-carbon bond, as shown in Figure 12-18.
The rate equation for an E2 reaction is as follows: rate = k[base][substrate]. Because the base is included in the rate equation, the base strength affects the rate of the reaction. The E2 reaction requires a strong base, and is the most common pathway for elimination reactions.
Seeing first-order elimination: The E1 reaction
First-order elimination, or the E1 reaction, is somewhat less common than second-order (E2) elimination. The mechanism for the E1 reaction, like the mechanism for the SN1 reaction, has two steps and is shown in Figure 12-19. First, the leaving group pops off to make the carbocation; this is the same first step as in the SN1 reaction. Then the base plucks off the hydrogen on an adjacent carbon to form the double bond.
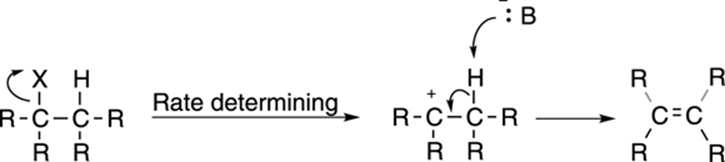
FIGURE 12-19: The E1 elimination mechanism.
Because the first step of the mechanism — the formation of the carbocation — is the rate-determining step, the rate follows the equation: rate = k[substrate]. Because the base is not included in the rate equation, the strength of the base is unimportant to the rate of the reaction. E2 elimination is favored with strong bases, so you see E1 elimination typically only with weak bases.
Help! Distinguishing Substitution from Elimination
Unfortunately, chemists live in an imperfect world. Reactions that are exclusively substitution or elimination are rare in the laboratory. Instead, chemists often get a mixture of the reaction products of both substitution and elimination reactions, because good nucleophiles generally are also good bases and vice versa. Here are a few generalizations, however, that will help you distinguish which of these reactions will predominate:
· Strong bases/nucleophiles force the reaction into second-order reactions. Thus, with strong bases and nucleophiles (such as OH–), you get SN2 or E2 reactions, or both. With weak bases/nucleophiles, you more often get first-order products (those produced by either SN1 or E1 reactions).
· Reactions of primary substrates generally proceed via SN2 reactions (methyl substrates always proceed by SN2). When very strong bases/nucleophiles are used with primary substrates, you get a mixture of both SN2 and E2 reactions.
· Reactions of tertiary substrates produce E1 and SN1 reactions with weak bases/nucleophiles plus a protic solvent; with strong bases, reactions of tertiary substrates produce E2 reactions.
· The reactions of secondary substrates are the hardest to predict. Under the right conditions, secondary substrates can undergo reactions by all four mechanisms. Weak bases/nucleophiles plus a protic solvent will typically give you a mixture of E1 and SN1 products; strong bases/strong nucleophiles will typically give you a mixture of E2 and SN2 products.
· Spotting nucleophiles that are not basic will help you distinguish substitution from elimination reactions. For example, the halides (I–, Br–, Cl–) and thiols (R-SH) are nucleophilic but not terribly basic. The reactions of these molecules typically proceed exclusively by substitution. t-Butoxide ((CH3)3CO–), on the other hand, is a poor nucleophile but a powerful base, and almost exclusively forces the reaction to go via an E2 elimination.