Organic Chemistry I For Dummies, 2nd Edition (2014)
Part V. The Part of Tens
Chapter 23. Ten Cool Organic Molecules
IN THIS CHAPTER
Seeing ten very cool organic molecules
Recognizing that organic molecules can be structurally and functionally interesting
In this chapter, I cover ten organic molecules that have structurally interesting features or turn out to be functionally useful. Or they just have structures that I think look cool.
Octanitrocubane
Organic explosives are molecules that usually combine both a source of fuel and an oxidizer within the same molecule, such that upon an initiating event (such as heat, shock, or light) an uncontrollable oxidation reaction occurs that generates pressurized gases, heat, and a resulting shockwave. Often, organic structures that contain both hydrocarbons (fuel) and oxidizing groups (such as nitro groups) can serve as explosives. Trinitrotoluene, or TNT, for example, contains both hydrocarbon fuel (the benzene ring) and oxidizing nitro groups within the same structure, leading to a compound with explosive properties.
An interesting recently developed explosive is octanitrocubane, shown in Figure 23-1. The idea behind this explosive is that the structure holds a great deal of energy in its highly strained rings. As a result, explosion of this molecule leads to more heat being given off when reaction converts the structure into acyclic small gases (such as N2, CO2, and so on) than if it were unstrained. Consequently, this molecule has the highest detonation velocity of known explosives. A drawback to this compound is that octanitrocubane is difficult to synthesize and, as a result, is very expensive compared to more common explosives.
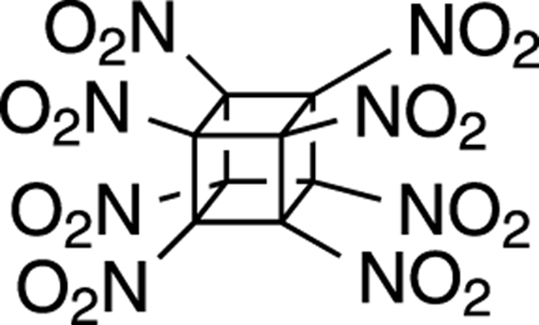
FIGURE 23-1: Octanitrocubane.
Fenestrane
Fenestrane (shown in Figure 23-2) is a cool-looking organic molecule that consists of four joined cyclobutane rings. This highly strained molecule is called fenestrane after the Latin word for window (fenestra), since the structure on paper resembles an old-fashioned windowpane.
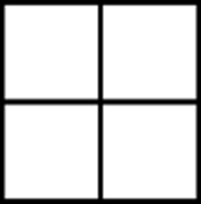
FIGURE 23-2: Fenestrane.
Carbon Nanotubes
Carbon nanotubes (shown in Figure 23-3) are a recently discovered allotrope of carbon, wherein planes of graphene (fused benzene ring sheets) are folded to form a cylinder. Carbon nanotubes have some extraordinary properties, including being the strongest materials known (many times stronger than steel) and having high thermal and electrical conductivities. New methods are still needed to make these materials on a large scale and in a cheaper way.
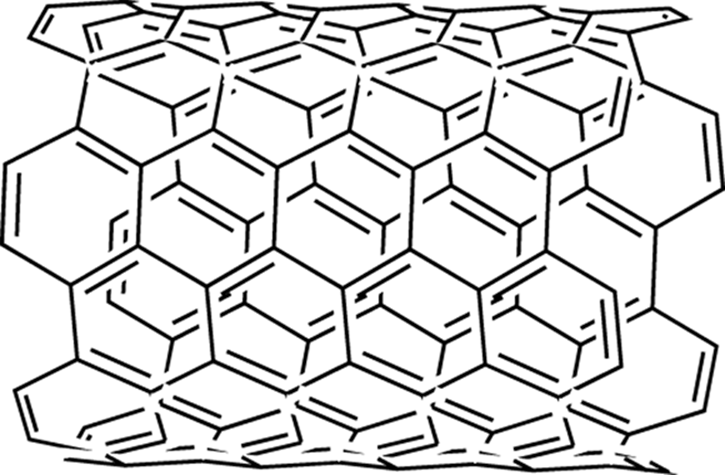
FIGURE 23-3: A small length of a carbon nanotube structure.
Bullvalene
Bullvalene is a really cool organic compound because it’s a molecular shape shifter. At room temperature, the molecule interconverts rapidly between numerous identical forms (the reaction mechanism is shown in Figure 23-4). A surprising consequence of these rapid rearrangements is that all ten carbons become equivalent in solution, leading this structure to have just a single peak in its 1H NMR spectrum at room temperature. At lower temperatures, the shape-shifting reaction is slowed and you can see the expected multiple proton absorptions in the 1H NMR spectrum. The molecule is named after William Doering (nicknamed “Bull” by his students), who did some pioneering studies on the molecule.
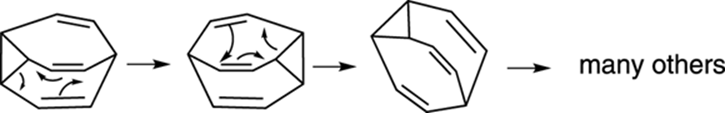
FIGURE 23-4: The shape-shifting molecule bullvalene.
The Norbornyl Cation
The determination of the structure of the norbornyl cation (shown in Figure 23-5) represents one of the biggest chemical controversies in the recent history of organic chemistry. On one side of the debate, Nobel laureate H. C. Brown thought that the norbornyl cation underwent a rapid equilibrium between two equivalent carbocation forms that interconverted by shifting atoms around. On the other side of the debate, Saul Winstein thought that the two forms of the norbornyl cation were actually resonance structures and that the norbornyl cation adopted a single “non-classical structure” that resembled a hybrid of the two cationic forms. The debate became heated at conferences and in the scientific literature, with both sides taking uncompromising stances. However, after much experimental and theoretical work, most scientists now believe that the structure of the norbornyl cation is the bizarre-looking non-classical form rather than a mix of rapidly interconverting isomers.
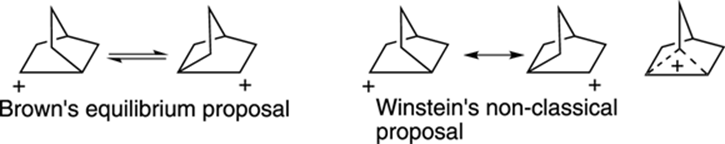
FIGURE 23-5: The norbornyl cation, now believed to adopt a non-classical structure.
Capsaicin
Capsaicin is a cool molecule because it gives a bit of spice to life — literally. Capsaicin is the main compound that gives hot peppers their heat. The Scoville unit, the most commonly used measure of how hot a pepper tastes, scales with the amount of capsaicin found inside a pepper. Note that the structure of capsaicin (shown in Figure 23-6) contains a long, greasy, hydrophobic tail, meaning that it doesn’t dissolve well in water. This gives rise to the oft-repeated advice to rinse your mouth out with milk (which contains a lot of non-polar fat) to reduce the burning sensation within your mouth rather than rinsing with water. The idea is that the hydrophobic fats within milk can solubilize the capsaicin better than water can. Capsaicin causes a burning sensation because it activates the same pain-signaling pathways as those activated by heat burns, making it feel as though your mouth were on fire.
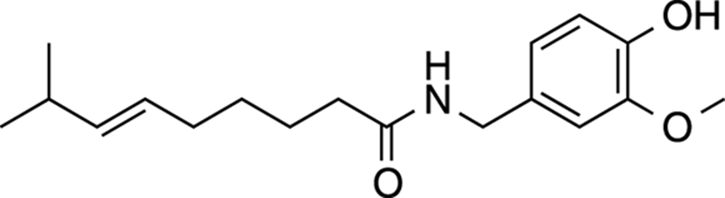
FIGURE 23-6: Capsaicin.
Indigo
Indigo (see Figure 23-7) is a blue compound that was initially isolated from indigo plants. It was considered a desirable compound because blue dyes were rare at the time it was isolated. Although the dye was originally extracted from plants and used industrially, today indigo is made synthetically by the metric ton and is used mostly to dye blue jeans.
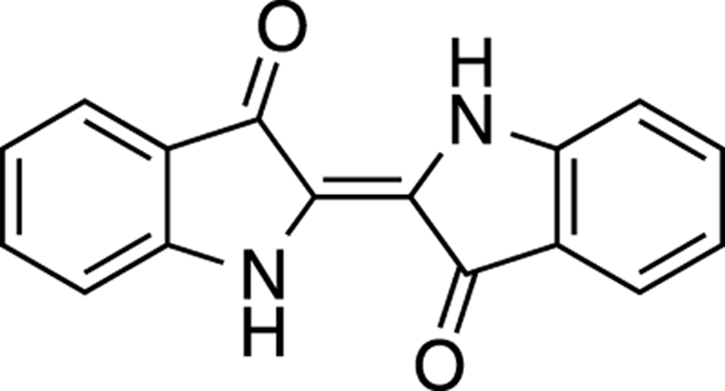
FIGURE 23-7: Indigo.
Maitotoxin
Maitotoxin is a beast of an organic molecule. This monstrous organic compound, containing 32 fused ring systems and 98 chiral centers (carbons with four different groups attached), was isolated from a tropical fish and is the most toxic “small” organic molecule known. The LD50 in mice for this compound is 50 ng/kg, meaning that a gram would probably be enough to kill hundreds of thousands of people. The proposed structure of maitotoxin is shown in Figure 23-8. If you can imagine trying to solve the structure of this compound using NMR spectroscopy or proposing a multistep synthesis to prepare the molecule in the lab, it makes you grateful for the (comparatively) easy problems you encounter in your organic chemistry class. There are current efforts underway to synthesize maitotoxin, and we can only wish the best of luck for all involved — they’ll probably need it.
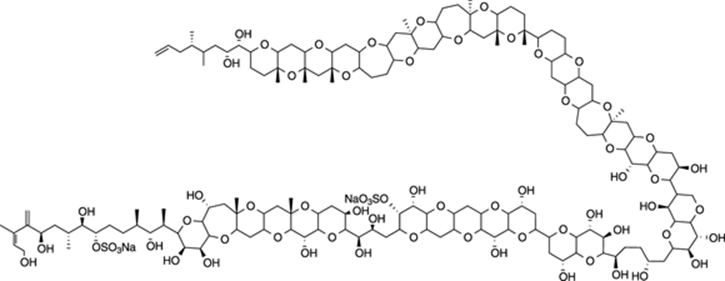
FIGURE 23-8: Maitotoxin.
Molecular Cages
Molecular cages are interesting organic structures that contain cavities that can encapsulate other atoms or molecules. A few examples are shown in Figure 23-9. The crown ether cages resemble crowns that can encapsulate metal ions (like K+) within the central cavity of the structure, while calixarenes resemble shuttlecocks that can encapsulate small organic molecules within their cavities. Cyclodextrins, which consist of a ring of connected glucose molecules, are found commercially in products like Febreze, where they can encapsulate volatile compounds with foul odors within their donut hole–like cavity, preventing you from smelling them. Some scientists are testing whether related molecular cages can act as drug delivery vectors for carrying toxic drugs to the site of a disease, such as bringing a chemotherapy molecule to the site of a tumor.
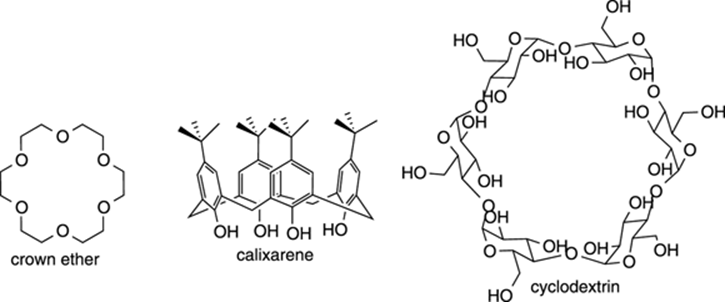
FIGURE 23-9: Some molecular cages.
Fucitol
Fucose is a naturally occurring deoxysugar (deoxy means that the structure is missing an OH group). Reducing the aldehyde of aldose sugars such as fucose using sodium borohydride makes a class of reduced sugars called alditols. The reduced form of fucose is, thus, called fucitol. This compound (shown in Figure 23-10) is found naturally in seaweed, and some people speculate that a dose of fucitol can change an attitude of anxiety, stress, overwork, and fatigue related to organic chemistry to a more Zen-like outlook on life.
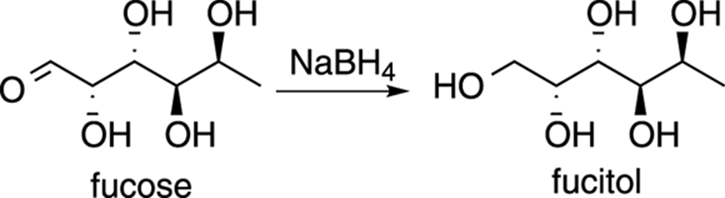
FIGURE 23-10: Fucitol. Consider when all else fails.