Organic Chemistry I For Dummies, 2nd Edition (2014)
Part I. Getting Started with Organic Chemistry
Chapter 4. Covering the Bases (And the Acids)
IN THIS CHAPTER
Defining acids and bases
Comparing acidities of organic molecules
Seeing pKa values
Predicting the directions of acid-base equilibria
You’ve surely done some acid-base chemistry in your lifetime, or at least observed some. Have you ever put lemon juice on fish to neutralize the fishy odors? Have you ever made a bottle rocket or fake volcano using baking soda and vinegar? Have you ever baked bread, cookies, or cakes? If so, then you’ve done acid-base chemistry. Certainly, you’ve dealt with acids and bases at some point. Foods such as tomatoes, oranges, lemons, sodas, and coffee are acidic, while household items such as bleach, ammonia, baking soda, and soaps are basic.
In fact, almost every reaction in organic chemistry involves acid-base chemistry — almost every one of them. Understanding how acids and bases work, therefore, is critical in understanding the reactions of organic molecules.
In this chapter, I define acids and bases using the three prominent definitions in use today. I show you how you can qualitatively predict the relative acidities of organic molecules by structural comparison, and I describe how organic chemists use a quantitative scale, called the pKa scale, to numerically define a molecule’s acidity. Then I show you how you can use this pKa scale to predict the direction of an acid-base reaction at equilibrium.
A Defining Moment: Acid-Base Definitions
Before I get to the nitty-gritty of how acids and bases work, I need to define what they are. Three definitions are currently accepted for defining whether a molecule is an acid or a base: the Arrhenius definition, the Brønsted-Lowry definition, and the Lewis definition.
Arrhenius acids and bases: A little watery
Svante Arrhenius, a prominent chemist from the early 20th century (whose ideas on acids and bases later earned him the Nobel Prize), defined acids as molecules that dissociate in water to make the hydronium ion, H3O+. Strong Arrhenius acids are those that completely dissociate in water to make hydronium ions, while acids that only partially dissociate in water are said to be weak Arrhenius acids. Nitric acid (HNO3), shown in Figure 4-1, is a strong acid because it completely dissociates in water to make hydronium ions; acetic acid (CH3COOH) only partially dissociates in water and is a weak acid. (The direction of the equilibrium in Figure 4-1 is shown with the larger arrow.)
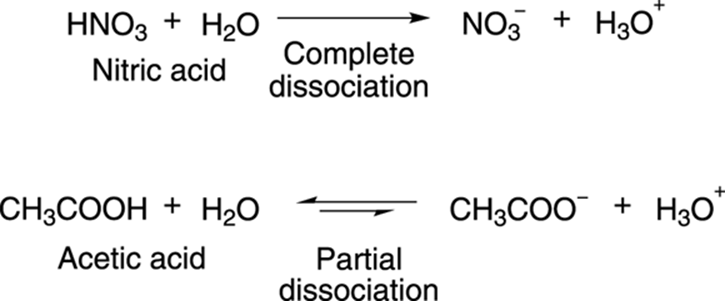
FIGURE 4-1: The dissociation of strong and weak acids.
Arrhenius bases, on the other hand, are molecules that dissociate to make hydroxide ions, OH–. As is the case with acids, bases that dissociate completely to generate hydroxide ions are strong bases, while bases that only partially dissociate to generate hydroxide ions are weak bases. Potassium hydroxide (KOH), shown in Figure 4-2, is a strong base because it completely dissociates in water to make hydroxide ions; beryllium hydroxide (Be[OH]2) is a weak base because it only partially dissociates in water.
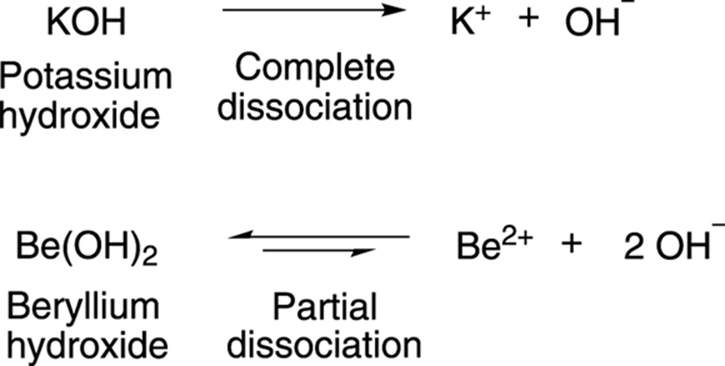
FIGURE 4-2: The dissociation of strong and weak bases.
Pulling for protons: Brønsted-Lowry acids and bases
The Arrhenius definition, though useful, has some “basic” problems. First, the definition can’t apply to all molecules, because many molecules aren’t soluble in water. Second, not all bases dissociate to generate the hydroxide ion. Ammonia (NH3), for example, creates hydroxide ion in solution but has no hydroxide ion in its formula, so it isn’t a basic molecule by virtue of dissociation.
Therefore, other acid-base definitions that are more widely applicable are necessary. The most commonly used acid-base definition in organic chemistry is the Brønsted-Lowry definition of acids and bases. A Brønsted-Lowry acid is a molecule that donates a proton (H+) to a base; aBrønsted-Lowry base is a molecule that accepts a proton from an acid.
 An H+ ion is called a proton because the hydrogen atom has no neutrons or electrons — just a single proton at the nucleus.
An H+ ion is called a proton because the hydrogen atom has no neutrons or electrons — just a single proton at the nucleus.
To keep the terminology straight, the deprotonated acid becomes what is known as the conjugate base (usually negatively charged, but not always), while the protonated base becomes the conjugate acid, as Figure 4-3 shows.
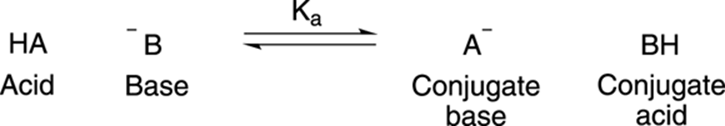
FIGURE 4-3: An acid-base reaction.
Electron lovers and haters: Lewis acids and bases
While the Brønsted-Lowry definition of acids and bases is more general than the Arrhenius definition, it still isn’t all-encompassing because it doesn’t include molecules such as BF3 or AlCl3, which neither donate protons nor accept them. The most general method for classifying acids and bases is the Lewis acid and base definition. A Lewis acid is a molecule that accepts a pair of electrons to make a covalent bond, and a Lewis base is a molecule that donates electrons to make a covalent bond.
Borane (BH3) is an example of a Lewis acid. Borane is a very unhappy molecule because it doesn’t have a full octet of valence electrons (see Chapter 2). Because it doesn’t have a full octet of electrons, it can accept a lone pair from a molecule like methylamine (CH3NH2), which has a lone pair of electrons, and use this electron pair to fill its octet (see Figure 4-4). Because BH3 accepts a pair of electrons to make a covalent bond, it’s said to be a Lewis acid; because methylamine donates electrons to make a bond, it’s said to be a Lewis base.
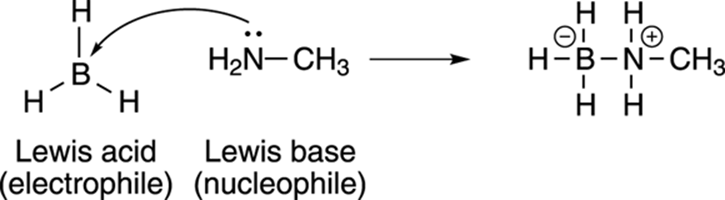
FIGURE 4-4: Lewis acids (electron acceptors) and Lewis bases (electron donors).
Lewis acids are also called electrophiles, which means “electron lovers.” Lewis bases are also called nucleophiles, which means “nucleus lovers.” You see the terms nucleophile and electrophile used repeatedly throughout organic chemistry courses.
 Nucleophiles are molecules that can donate electrons (Lewis bases) to form bonds and are nucleus lovers; electrophiles are molecules that can accept electrons (Lewis acids) to make a new bond and are electron lovers.
Nucleophiles are molecules that can donate electrons (Lewis bases) to form bonds and are nucleus lovers; electrophiles are molecules that can accept electrons (Lewis acids) to make a new bond and are electron lovers.
Lewis acids and bases encompass Brønsted acids and bases as well. Any Brønsted acid will also be a Lewis acid, and any Brønsted base will also be a Lewis base (see Figure 4-5 for an example). You may reasonably ask, “If that’s the case, why use Brønsted acids and bases at all?” The answer is that it’s often more convenient for the organic chemist to think of acid-base reactions in terms of proton transfers rather than in terms of electron transfers. That’s why when acids and bases are discussed in organic chemistry, with only a few exceptions, it’s in terms of Brønsted acid and bases.
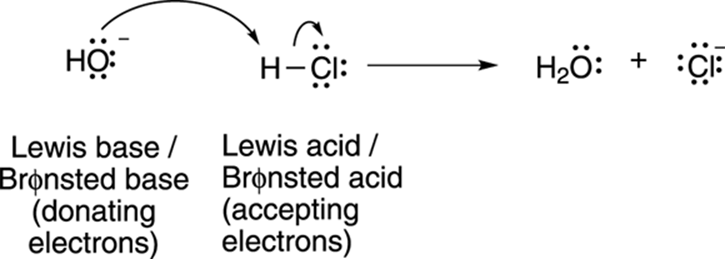
FIGURE 4-5: Brønsted acids are also Lewis acids.
A WORD ABOUT ACIDS AND BASES
It’s easy to call a molecule an acid or a base, and say, “That’s all there is to it, folks.” But the terms acid and base are a little more elusive. A molecule is an acid only in comparison to another molecule, and likewise for a base. When the terms acid or base are used when discussing a particular molecule, they’re used in comparison to a reference molecule — water. Water is capable of acting both as an acid and as a base. Any molecule that’s more acidic than water is generally considered an acid, and any molecule that’s more basic than water is generally considered a base.
But keep in mind that these terms are general. Most people would agree that nitric acid is an acid; its name even includes the word acid. But even nitric acid can act as a base under the right conditions! In the presence of the more acidic sulfuric acid, nitric acid acts as a base (you see this reaction in the nitration of benzene in Chapter 15). This reaction is an extreme case, but I hope it makes you wary of rigidly classifying a molecule as an acid or a base, even though doing so is convenient. Whether a molecule acts as an acid or as a base really depends on what’s thrown into the reaction pot along with it.
Comparing Acidities of Organic Molecules
In general, the strength of an acid is directly proportional to the stability of the acid’s conjugate base. In other words, an acid that has a more stable conjugate base will be more acidic than an acid that has a less stable conjugate base. Because the conjugate base is usually negatively charged (anionic), a convenient tactic when comparing the acidities of molecules is to think about what structural features contribute to stabilizing negative charges. The more stable these negative charges are in the conjugate base, the more acidic the acid. In the sections that follow, I talk about structural features that stabilize negative charges and lead to stronger acids.
 Strong acids have stable conjugate bases.
Strong acids have stable conjugate bases.
In general, acidic molecules have structural features that allow the anion in the conjugate base to delocalize the charge over a larger space. Delocalization of the negative charge (such that one atom doesn’t have to bear the full negative charge) makes the molecule more stable. The most important features that stabilize negative charges include the electronegativity, hybridization, and size of the atom upon which the negative charge is located, the electron-withdrawing effects of neighboring electronegative atoms, and resonance effects.
Comparing atoms
On which atom does the negative charge of the acid’s conjugate base rest? A negative charge prefers to rest on electronegative (electron-loving) elements. Therefore, a negative charge is more stable on oxygen than it is on nitrogen; similarly, a negative charge is more stable on nitrogen than it is on carbon. For that reason, alcohols (R—OH) are more acidic than amines (R—NH2), which in turn are more acidic than alkanes (R—CH3), as shown in Figure 4-6.
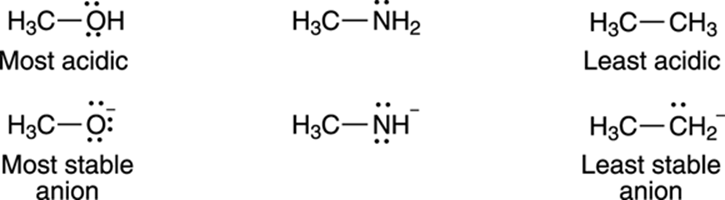
FIGURE 4-6: Negative charges prefer to rest on the more electronegative atoms.
 Electronegativity increases as you go up and to the right on the periodic table.
Electronegativity increases as you go up and to the right on the periodic table.
The size of the atom also plays a role in stabilizing the negative charge. Charges prefer to be on larger atoms than on smaller atoms. This preference results from large atoms allowing the negative charge to delocalize over a much larger region of space, instead of being concentrated in a small region (as it would on a small atom). As a rule, atom size trumps electronegativity considerations. So, even though fluorine is a more electronegative atom than iodine, HI is more acidic than HF. The much larger iodine atom allows the negative charge to delocalize over a larger space than does the much smaller fluorine atom, and thus makes hydrogen iodide more acidic. The effect that atom size has on acidity is shown in Figure 4-7.
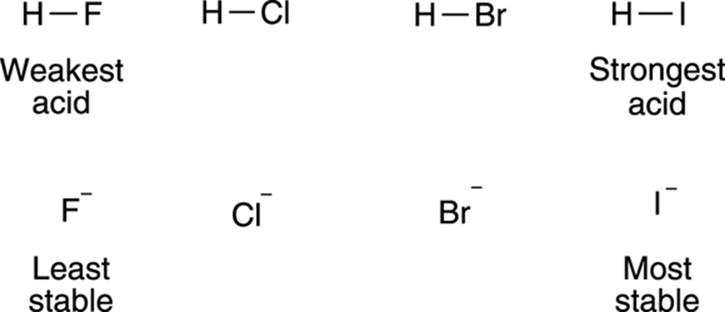
FIGURE 4-7: The size of the atom contributes to acidity.
Seeing atom hybridization
The orbital on which the lone-pair anion rests also affects the acidity. Lone-pair anions prefer to reside in orbitals that have more s character than p character, because s orbitals are closer to the atom’s nucleus than p orbitals, and the electrons are stabilized by being closer to the nucleus. Orbitals that are sp hybridized have 50 percent s character, orbitals that are sp2 hybridized have 33 percent s character, and orbitals that are sp3 hybridized have 25 percent s character. Therefore, anions prefer to be in orbitals that are sp hybridized over those that are sp2 hybridized orbitals, and they prefer to be in orbitals that are sp2 hybridized over those that are sp3 hybridized. The effect of orbital type on acidity is shown in Figure 4-8. (Refer to Chapter 2 to read more about orbital hybridization.)
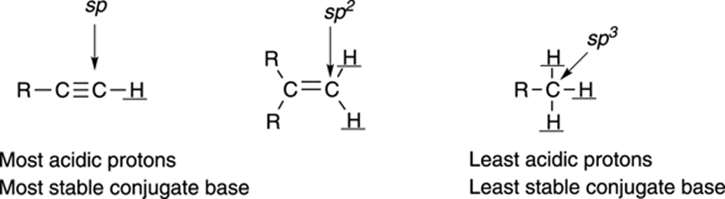
FIGURE 4-8: The types of orbitals affect acidity.
Seeing electronegativity effects
Electron-withdrawing groups on an acid also stabilize the conjugate base anion by allowing some of the charge on the anion to delocalize to other parts of the molecule. For example, trifluoroethanol (shown in Figure 4-9) is more acidic than ethanol. The highly electronegative fluorine atoms (which are electron pigs) on trifluoroethanol pull electron density away from the anion, taking away some of the negative charge from the oxygen, thereby stabilizing the molecule.
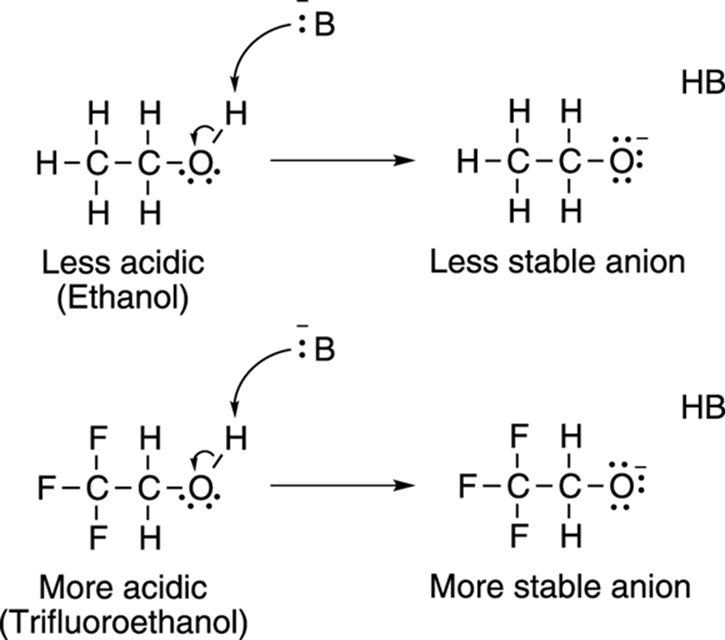
FIGURE 4-9: Electron-withdrawing groups add to a molecule’s acidity by stabilizing its conjugate base anion.
Seeing resonance effects
Acids with conjugate bases that allow the negative charge to be delocalized through resonance are stronger acids than acids whose conjugate bases don’t have resonance structures. For example, acetic acid, shown in Figure 4-10, is much more acidic than ethanol because the conjugate base anion of acetic acid can delocalize the negative charge through resonance.
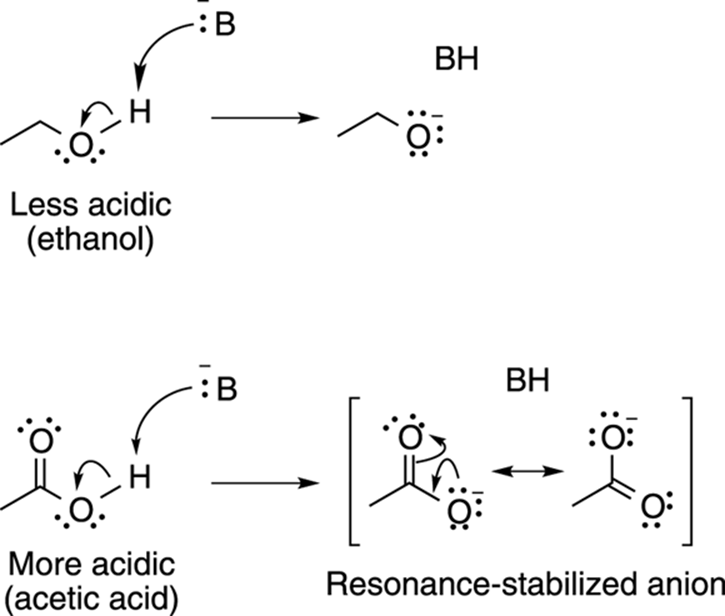
FIGURE 4-10: Resonance effects contribute to acidity.
 Resonance structures are a stabilizing feature of molecules and ions.
Resonance structures are a stabilizing feature of molecules and ions.
Defining pKa: A Quantitative Scale of Acidity
The pKa value of an acid is a quantitative measurement of a molecule’s acidity. The pKa is derived from the equilibrium constant for the acid’s dissociation reaction, Ka, and uses a logarithmic scale to allow the pKa values to span wide ranges.
pKa = –log Ka
 The lower the pKa value of an acid, the stronger the acid. The higher the pKa value, the weaker the acid. Very strong acids have pKa values of less than zero, while weak acids generally have pKa values of between 0 and 9.
The lower the pKa value of an acid, the stronger the acid. The higher the pKa value, the weaker the acid. Very strong acids have pKa values of less than zero, while weak acids generally have pKa values of between 0 and 9.
A brief pKa table of acids is shown in Table 4-1.
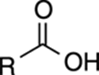
TABLE 4-1 Approximate pKa Values of Common Acids
|
Acid |
Approximate pKa |
Acid |
Approximate pKa |
|
H2SO4 |
–7 |
H3O+ |
–2 |
|
HCN |
9 |
|
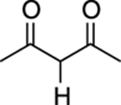
5 |
|
R–OH |
16 |
|
10 |
|
|
20 |
CH4 |
50 |
Problem Solving: Predicting the Direction of Acid-Base Reactions at Equilibrium
With pKa table in hand (or in memory, if your professor insists upon it), you can predict the equilibrium direction of acid-base reactions. Weak acids and bases are lower in energy than strong acids and bases, and because equilibria favor the reaction side with the lowest-energy species, acid-base reactions will go to the side with the weakest acids and bases.
 As a rule, the equilibrium of a reaction will favor the side with weaker acids and bases.
As a rule, the equilibrium of a reaction will favor the side with weaker acids and bases.
For example, you can predict the direction of the acid-base reaction between hydrogen cyanide (HCN) and acetate (C2H3O2–) shown in Figure 4-11. Because hydrogen cyanide (pKa = 9) has a higher pKa value than acetic acid (pKa = 5), the equilibrium will lie to the left, in the direction of the weaker acid and base. That’s really all there is to predicting the direction of an acid-base reaction. If you know the pKa values of the two acids on both sides of the equation, then you know in which direction the equilibrium lies, because equilibrium will favor the side with the acid that has the highest pKa.
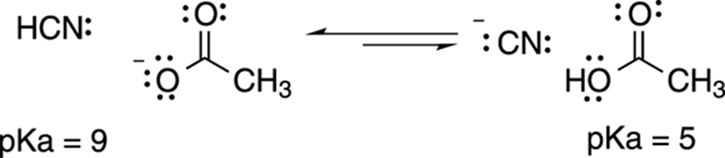
FIGURE 4-11: The pKa values predict the direction of the acid-base equilibrium.