Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Oxidation Reactions in Organic Chemistry
11.2 Oxidation
Combustion is the reaction of compounds with oxygen under suitable reaction conditions. Reaction (11-1) shows the combustion of methane, and Reaction (11-2) shows the combustion of butane.
(11-1)![]()
(11-2)![]()
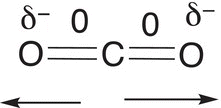
Complete combustion of alkanes yields carbon dioxide and water as the products. These reactions are very important, during the winter, and the combustion of natural gas is used to heat our homes, classrooms, and workplaces. Natural gas is 60—90% (methane) and the other 10—40% consists of C2H6 and C3H8, N2, CO2. A great deal of natural gas is found in the Texas Panhandle and Oklahoma of the United States. Petroleum, also called crude oil, is a mixture of aliphatic and aromatic compounds and a small percentage of sulfur and nitrogen compounds. There are as many as 500 different compounds present in petroleum or crude oil. This crude product from the ground is not very useful since it consists of so many different compounds. However, refining — the process of distillation — separates the different components of crude oil into various fractions. One fraction is gasoline, which as we know is used as the fuel for the combustion engine. This fraction when used as fuel, however, causes knocking in engines, and to prevent knocking, sometimes other alkanes and aromatic compounds are added to the fuel. It was observed that 2,2,4-trimethylpentane (isooctane) could be used as an antiknocking compound for gasoline engines. As a result, it is used to rate fuels. Examples of the amount of heat liberated from the combustion of different alkanes are given in Reactions.
(11-3)![]()
(11-4)![]()
(11-5)![]()
(11-6)![]()
The negative sign indicates that heat is released from the combustion, i.e. these are all exothermic reactions.
Problem 11.1
Give a balanced equation for the combustion of hexane.
Oxidation reactions involving oxygen are easy to recognize since oxygen is the oxidizing agent. Another definition of oxidation is that there is a loss of hydrogen. For the reaction given in Reaction (11-1) in which methane is oxidized, methane (CH4) loses hydrogen atoms to become CO2, and hence meets the requirement of being oxidized since it loses hydrogen atoms in going from reactant to product.
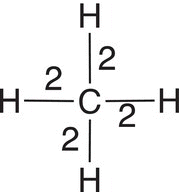
The other definition of oxidation states that there is a loss of electrons. In order to determine a loss of electrons, we must determine the oxidation state of the carbon of the reactant and also in the product. Let us consider the oxidation of methane (Reaction 11-1). In order to determine the oxidation state of carbon in the reactant (methane), we will have to start by looking at the oxidation state of elemental carbon, and how many valence electrons are associated with this atom. You will recall that there are four valence electrons associated with elemental carbon and since it is an element, it is assigned an oxidation state of zero. Since carbon is bonded to other atoms, typically by covalent bonds, we will have to take into consideration the electrons from the other bonding atoms. In the case of methane, they are four hydrogen atoms. Shown in Table 11.1 is the calculation for the oxidation of the carbon of methane and carbon dioxide. For methane, there are eight electrons that are shared in the bonding to methane and they are used in the calculation and shown in the second column, last line. For the product, CO2 shown in Reaction 11-1, the number of electrons considered for the calculation is zero since these bonding electrons are closely associated with the electronegative oxygen, compared to the carbon. Thus, the oxidation state of the carbon in carbon dioxide is positive four (+4), as shown in the calculation in Table 11.1 (last column, last line).
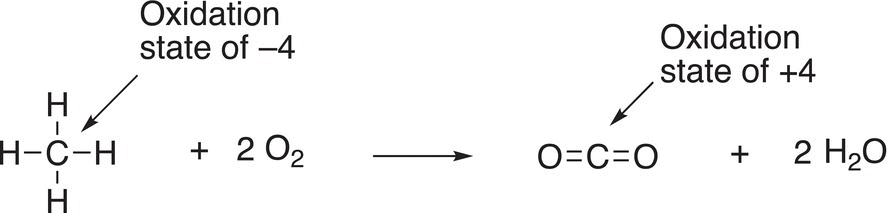
Table 11.1 Calculating the oxidation states for methane and carbon dioxide.
Molecule |
|
|
|
Number of electrons used for calculation |
4 |
8 |
0 |
Oxidation state |
0 |
4 − 8 = −4 |
4 − 0 = +4 |
Therefore, for the combustion reaction shown in Reaction (11-1), the carbon of methane has an oxidation state of −4 and is transformed into carbon dioxide via this reaction, with an oxidation state of +4. This involves a loss of electrons, hence oxidation. We will see more examples in the next section when we look at the reactions of more complex molecules, but the same concept of determining oxidation states can be applied.
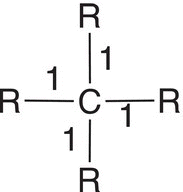
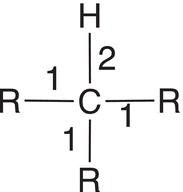
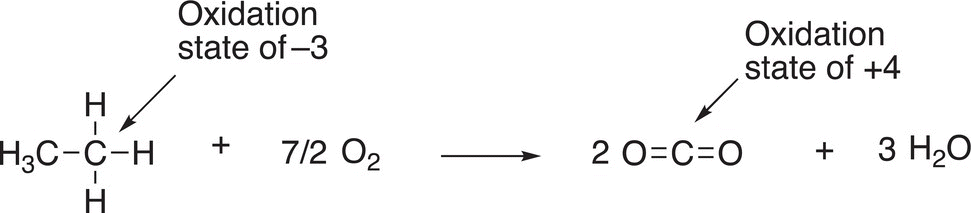
Let us now consider a molecule, where all four hydrogens of methane are substituted with alkyl groups, such as methyl groups. The bonding electrons are now equally shared since both atoms are carbon atoms with the same electronegativity. Thus, there are four electrons associated with the central carbon in this case and not eight as was the case with methane. As a result, the oxidation state of 2,2-dimethylpropane is zero (0) as shown in the calculation in Table 11.2, second column, last row. The other example shown in the table has one hydrogen atom and three alkyl groups bonded to the central carbon. As shown in the last column, there are now five electrons associate with this calculation giving an oxidation state of negative one (−1) as shown in the last column, last row.
Thus, for the oxidation of ethane, the oxidation state of the carbon shown below is −3; in the product, the oxidation state of the carbon of carbon dioxide is +4. Since the carbon goes from negative to positive, ethane has been oxidized.
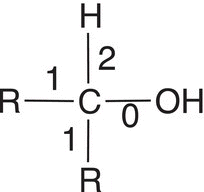
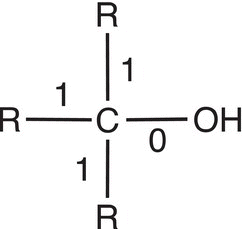
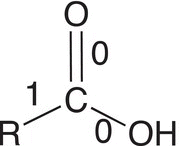
Let us now consider the oxidation states of carbons of molecules that are bonded to heteroatoms, such as oxygen, which are more electronegative than carbon. As a result, the covalent bonding electrons will all be associated with the heteroatom and not with carbon, and the method of counting bonding electrons is the same as with carbon dioxide. Table 11.3 gives examples of secondary and tertiary alcohols and carboxylic acids.
Table 11.2 Calculation the oxidation states for various types of hydrocarbons.
Molecule |
|
- |
|
Number of electrons used for calculation |
4 |
4 |
5 |
Oxidation state |
0 |
4 − 4 = 0 |
4 − 5 = −1 |
Table 11.3 Calculation the oxidation states for molecules with oxygen.
Molecule |
|
|
|
Number of electrons used for calculation |
4 |
3 |
1 |
Oxidation state |
4 − 4 = 0 |
4 − 3 = +1 |
1 − 4 = +3 |
Reactions (11-7)—(11-9) give examples of oxidation reactions, in which the oxidation states of the carbons in both the reactants and products are shown.
(11-7)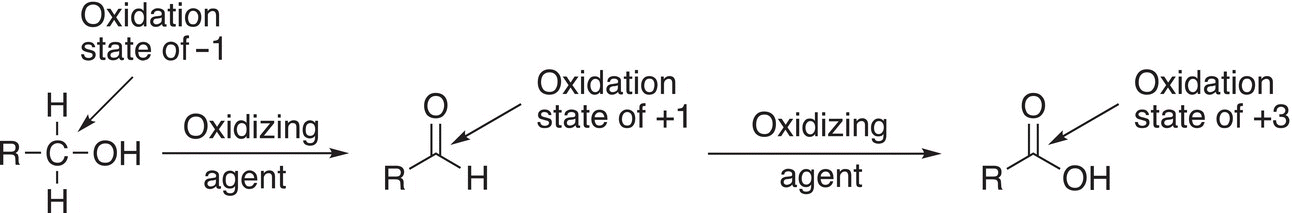
(11-8)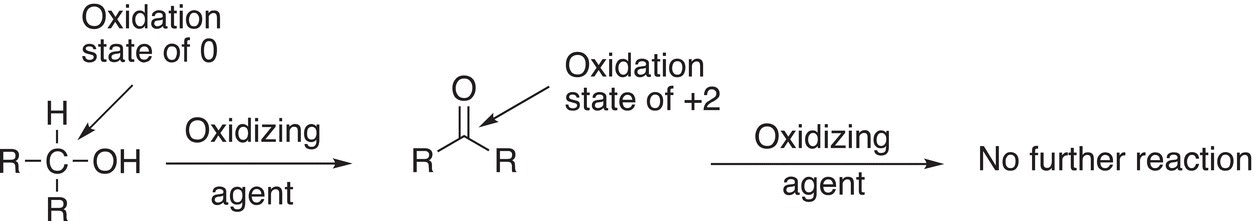
(11-9)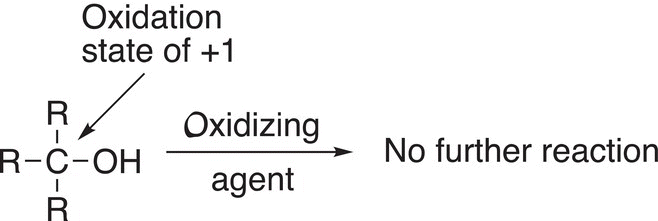
For Reaction (11-7), note that the reactant is a primary alcohol, and it is oxidized initially to an aldehyde, which can be oxidized further to the carboxylic acid. Note that since the aldehyde has one hydrogen atom bonded to the carbon of the carbonyl, it can be oxidized further by losing that hydrogen and of course a further loss of electrons. For Reaction (11-8), the reactant is a secondary alcohol and is oxidized to a ketone. Note that the ketone cannot be oxidized further since it does not have a hydrogen bonded to the carbonyl carbon. The same is true for tertiary alcohols; for Reaction (11-9), there is no reaction since a tertiary alcohol cannot be oxidized further since it does not have a hydrogen atom bonded to the carbon of the alcohol functionality.
Problem 11.2
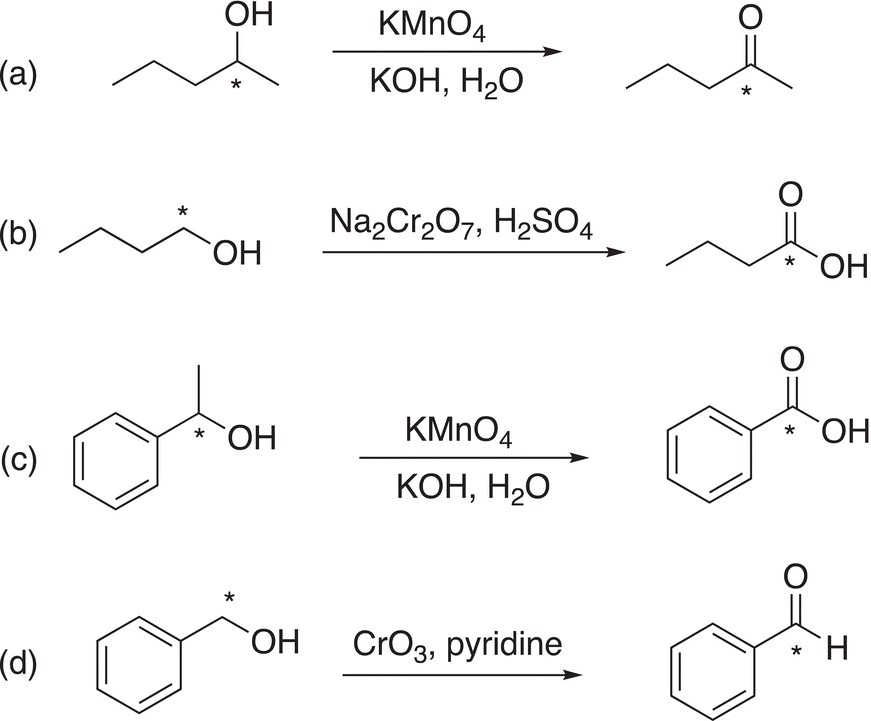
i. Give the oxidation state of the starred carbons for each of the following molecules.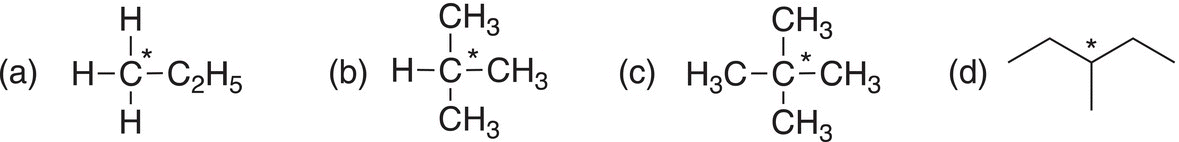
ii. Give the oxidation state of the starred carbons for each of the following molecules.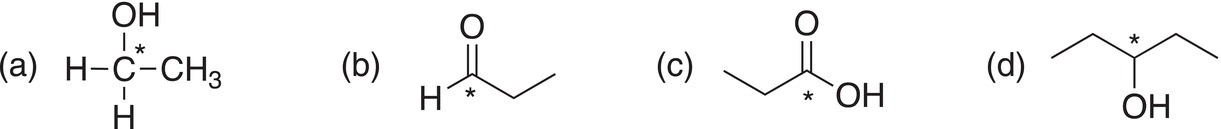
iii. For the reactions below, determine the oxidation state of the starred carbons of the reactants and products and determine if an oxidation occurred.