Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Aromaticity and Aromatic Substitution Reactions
17.4 Stability of Benzene
It was mentioned earlier in the chapter that benzene is often used as a solvent in industrial and academic research labs. Solvents are used in reactions to provide an inert medium so that the reactions can take place; that is, they do not normally react with the reactants or the products. Since benzene is a commonly used solvent, the implication is that it is a very stable and an inert molecule. To fully understand why benzene is inert, let us examine the heat liberated upon hydrogenation of benzene compared to similar compounds. For a particular compound, the amount of heat liberated upon hydrogenation can be used to give a very good approximation of the stability, compared to similar compounds. We used this concept when we examined the stability of the different isomers of a particular alkene in Chapter 4.
For different compounds that form the same product by the same type of reaction, the reactant that liberates the most heat is the least stable, as illustrated in Figure 17.11.
The hydrogenation of cyclohexene, cyclohexadiene, cyclohexatriene (not a real molecule), and benzene all give the same product, cyclohexane. The hydrogenation of cyclohexene is an exothermic reaction and 28.6 kcal mol−1 of heat is liberated, as given in Reaction (17-3).
(17-3)

Figure 17.11 Energy diagram illustrating the relationship between the stability of reactants that give the same product, via the same type of reaction.
The molecule with two double bonds that are not in conjugation with each other (1,4-cyclohexadiene) liberates approximately twice as much heat, as the hydrogenation of cyclohexene Reaction (17-4).
(17-4)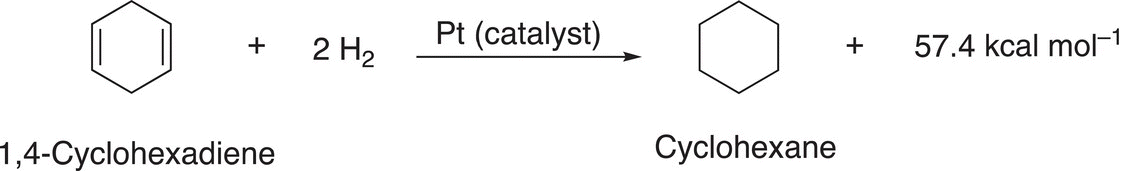
The hypothetical molecule with three double bonds in a cyclic six-member ring, cyclohexatriene, which is not a real molecule (one of Kekulé's incorrect structures of benzene) is expected to liberate three times the amount of heat upon hydrogenation as that liberated from hydrogenation of cyclohexene. The hypothetical reaction is shown in Reaction (17-5).
(17-5)
Note that 85.8 kcal mol−1 of heat is the predicted value for the hypothetical cyclohexatriene molecule, which is three times that for cyclohexene. This prediction is reasonable since the actual heat liberated from 1,4-cyclohexadiene (which has two double bonds) is twice that of cyclohexene. Benzene, the real molecule, liberates only 49.8 kcal mol−1 of heat as shown in Reaction (17-6).
(17-6)
The energy profile for these reactions is shown in Figure 17.12, which shows benzene to liberate the least amount of heat.
It is obvious from these results that benzene is more stable than expected when compared to the hypothetical cyclohexatriene. Cyclohexatriene is expected to liberate 85.8 kcal mol−1 of heat, but the real molecule, benzene, yields only 49.8 kcal mol−1 upon hydrogenation. Thus, benzene is 36.0 Kcal/mol more stable than expected (85.8—49.8 kcal mol−1). This energy, 36.0 kcal mol−1, is also called the resonance energy of benzene. Since benzene is much more stable than expected, this observation explains why it can be used as a solvent and is fairly nonreactive.
ΔΔ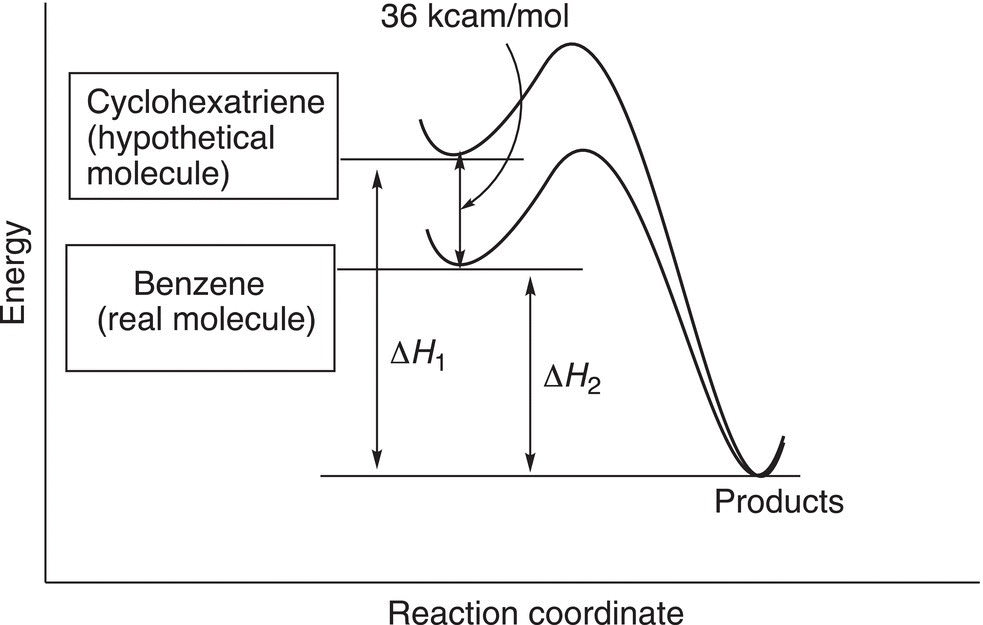
Figure 17.12 Representation of the exothermic reactions of benzene and the hypothetical, 1,3,5 cyclohexatriene.