Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Heteroatomic Functional Groups and Organic Nomenclature
3.6 Nomenclature of Ketones
Compounds that have the ketone functionality are named as alkanones. The -ane of the corresponding alkane root name is changed to -anone (or the e of the corresponding alkane root name is changed to one). In naming alkanones, the longest continuous chain that contains the carbonyl functionality is identified and based on the number of carbons present, the root name of the alkanone is determined. In naming ketones, the carbon that gets number 1 is the carbon that is closest to the carbonyl functionality and a number must be used to specify the position of the carbonyl functionality of the ketone, as shown in the example below.
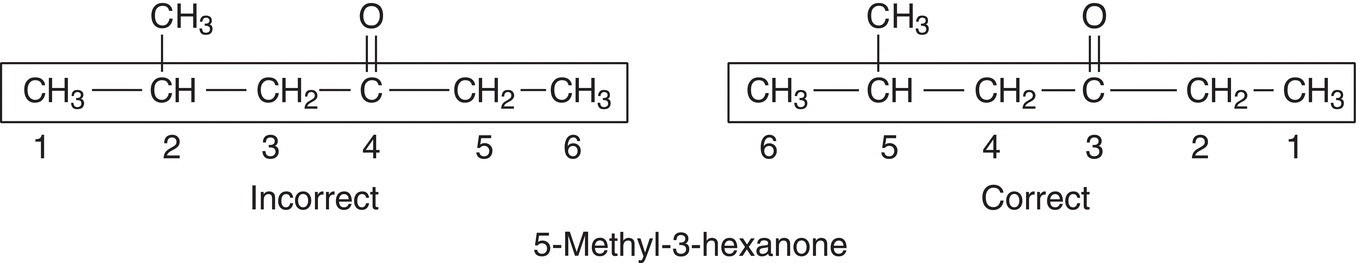
Note that in the above incorrect example, even though the branch is in position 2 and the ketone functionality is in position 4, the branch does not take priority over the carbonyl functionality for the assignment of numbers in naming ketones. That is, the carbonyl functionality must always get the lowest number and not the location of the branch. In the above correct example, the number 3 is used to specify the position of the carbonyl functionality.
3.6.1 Nomenclature of Difunctional Ketones
A source of confusion in the nomenclature of ketones comes when there is another functionality present in the same molecule. If the other functionality is an alkene, for example, the position of the alkene functionality is specified based on the numbering system of the root name given to the alkanone, as shown in the example below.
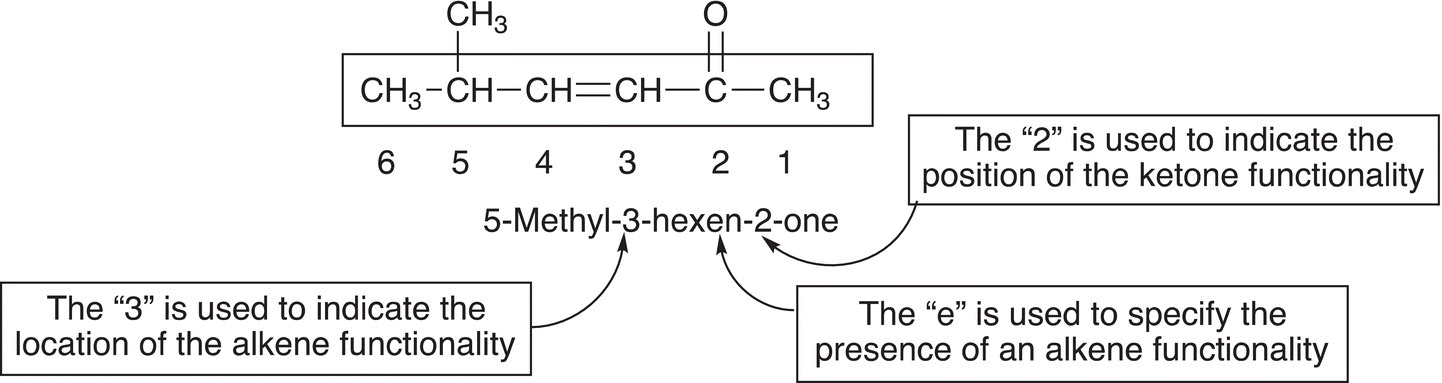
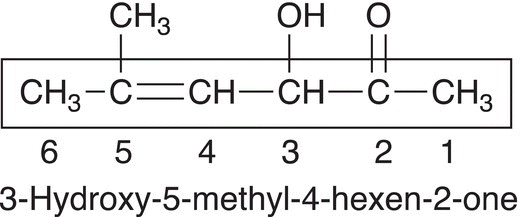
If an ─OH functionality is present in the same molecule that contains a ketone functionality, a number is used to indicate the presence of the ─OH functionality and the molecule is named as a hydroxy alkanone (and not as an alkanol). For the compound below, which has three functionalities, note that the numbering is based on the presence of the ketone, and not on the presence of the ─OH or alkene functionalities. The ketone takes priority over the branching, an alkene, or an alcohol functionality.
Problem 3.10
i. Give the structures of the compounds shown below.
1. 3-Hydroxy-2-pentanone
2. 4-Methyl-2-pentanone
3. 3,3-Dimethyl-2-butanone
4. 5-Methyl-3-hexen-2-one
5. 3-Pentyn-2-one
ii. Give IUPAC names for the compounds shown below.

3.6.2 Nomenclature of Cyclic Ketones
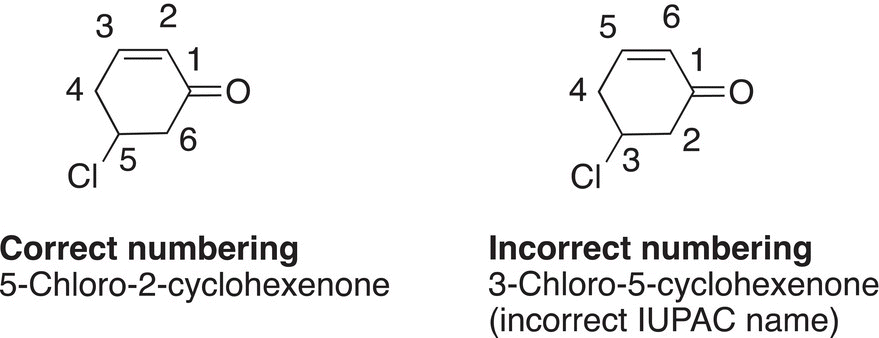
In naming cyclic alkanones, the root name is derived from the corresponding cyclic alkane and the cycloalkane becomes cycloalkanone. In numbering cycloalkanones, the carbon that has the carbonyl functionality gets #1. Examples are shown below.
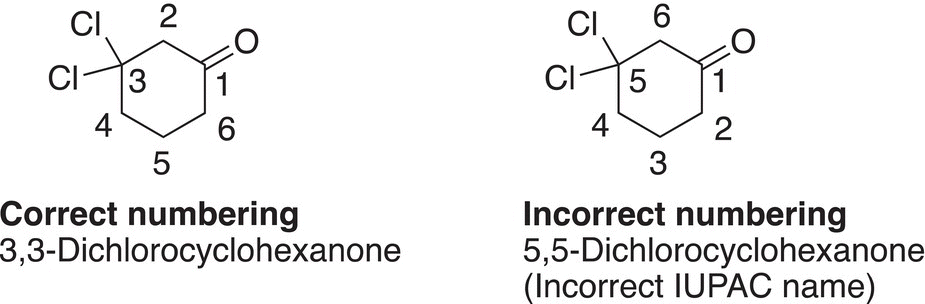
Note that the numbering from position #1 proceeds in the direction of the closest branch or functionality. Shown below is an example of a cyclic ketone, which also contains an alkene functionality.
Problem 3.11
i. Give line-angle structures of the following molecules.
1. 2,3-Dichloro-4-methylcyclopentanone
2. 5-Methyl-3-hexanone
3. CH3C(CH3)2CH2COCH2CH3
ii. Give the IUPAC name of the molecule shown below.
