Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Bonding and Structure of Organic Compounds
1.2 Electronic Structure of Atoms
Before we actually examine how atoms are bonded together to form different organic molecules, we need to review our understanding and concept of atoms, and more specifically, at the electronic level. There are four elements that we will encounter frequently throughout our study of organic chemistry: hydrogen (H), carbon (C), nitrogen (N), and oxygen (O). You should locate these elements on the periodic table before continuing. Note carefully their location on the periodic table in relationship to each other and the various numbers that are associated with each atom. First, we will review the electronic structure of these atoms. It is extremely important that students and instructors as well as other scientists across the world visualize the structure of each atom and compounds similarly in order to effectively communicate concepts and explain various scientific observations. Studying organic chemistry is like learning a new language; if we are to learn a new language, we will have to learn the basics, such as the alphabet and symbols in order to make sure that there is a universal understanding of the language so that it can be utilized effectively to communicate with others across the world.
Thus, the first part of this course focuses on gaining a universal concept and visualization of the three-dimensional description of atoms and molecules. It is extremely important to stress the three-dimensional visualization since our understanding and explanations of different observations will depend on our three-dimensional concept of atoms and molecules. Reactions of molecules take place in a three-dimensional world and hence it is important that we visualize molecules from that perspective. The use of molecular model sets will be very helpful to better visualize atoms and molecules in three dimensions. Once students gain a good understanding of the three-dimensional world of atoms and molecules, it becomes much easier to communicate chemistry concepts on a two-dimensional paper.
1.2.1 Orbitals
The simplest description of the atom is that it consists of neutrons, protons, and electrons, and this simple description is enough for us to appreciate and understand most of the concepts of organic chemistry. The question now becomes, where are these electrons, protons, and neutrons in the atom? We know from general chemistry that the nucleus contains the neutrons, which are neutral, and also the protons, which are positively charged, but where are the electrons? Electrons are not randomly distributed throughout the atom, but they are located in specific regions of the atom. Thus, our first task is to get a picture of the structure of the atom and then try to visualize the location of the electrons. A comparison that can be used to gain a visual description of how electrons populate the atom is to make the comparison of students populating a large dorm of a college or university. In a dorm, there are many rooms and different types of rooms, i.e. the bedroom, bathroom, living room, and so on. Once a dorm is built, the next task is to get students to occupy the dorm. It is very important to ensure that as students start to occupy the dorm, the dorm is occupied properly based on rules established by the university. For example, one of these rules could be that students must occupy the first floor before occupying the second and third floors, and so on.
Regarding the structure of the atom, there are no rooms, bathrooms, and so on, like you would find in a dormitory; but in addition to the nucleus, there are regions outside the nucleus called orbitals. The nucleus is a spherical tiny space, which contains the protons and neutrons and is located in the center of the atom. Orbitals are another region of the atom, which are located outside the nucleus, and an orbital is the region where electrons occupy. There are various types of orbitals like there are various types of rooms in a dormitory, and we will discuss each type later in the chapter. In order to fully appreciate how electrons are distributed within the atom, we need to get a good concept of orbitals and their location around the nucleus. The nucleus of an atom is very small and is approximately 10−14 m in diameter, and the diameter of an entire atom, including the electrons, is approximately 10−10 m. Although it is impossible to determine the exact location of the electrons in an atom, it is possible to gain a good approximation of the region in space where the electrons are most likely to be found. There is a greater probability of finding the electrons closer to the nucleus than further from the nucleus (remember that the nucleus contains the positively charged protons). The electrons are not randomly distributed in the space outside the nucleus, but they are most likely to be found in specific regions of space called orbitals. A comparison could be made with students in the dormitory; sometimes, it is almost impossible to tell exactly where in the dormitory a specific student is located, but around 2:00 in the morning, we could say that there is a good probability of finding the student in the room sleeping!
Orbitals have different sizes and shapes, similar to dormitory rooms that have different sizes and shapes. For atoms, numbers are used to represent orbitals of different energies, and letters are used to represent orbitals of different shapes. The s orbitals are spherical, and there are s orbitals that are larger than other s orbitals. Principal quantum numbers are used to differentiate between s orbitals of different sizes and energy. A small principal quantum number that is associated with the letter s suggests that the s orbital is smaller and close to the nucleus, and hence low in energy, compared to another s orbital that has a larger principal number, which would be larger and higher in energy and hence further from the nucleus. Thus, the 1s orbital is small, close to the nucleus, and low in energy, compared to the 2s orbital, which is larger, further from the nucleus, and higher in energy as illustrated in Figure 1.1
The s orbitals are not the only type of orbitals that are present in an atom, there are also other types of orbitals, and the p orbitals are of another type. The shape of p orbitals is different from the spherical shape of the s orbitals. The shape of the p orbitals is often described as dumbbell shaped or as that of an hourglass, as shown in Figure 1.2.
Thus, for these orbitals, the probability of finding the electrons is not in a spherical region of space as that for the s orbitals, but only in the three-dimensional region that is outlined by the geometry of the shape of the orbitals. As shown in Figure 1.2, some p orbitals are small and close to the nucleus and other p orbitals are larger and further from the nucleus. Principal quantum numbers are used also to describe the relative size of different p orbitals, relative energy, and distance from the nucleus. Thus, a 2p orbital is smaller than a 3p orbital; note that there is no 1p orbital.
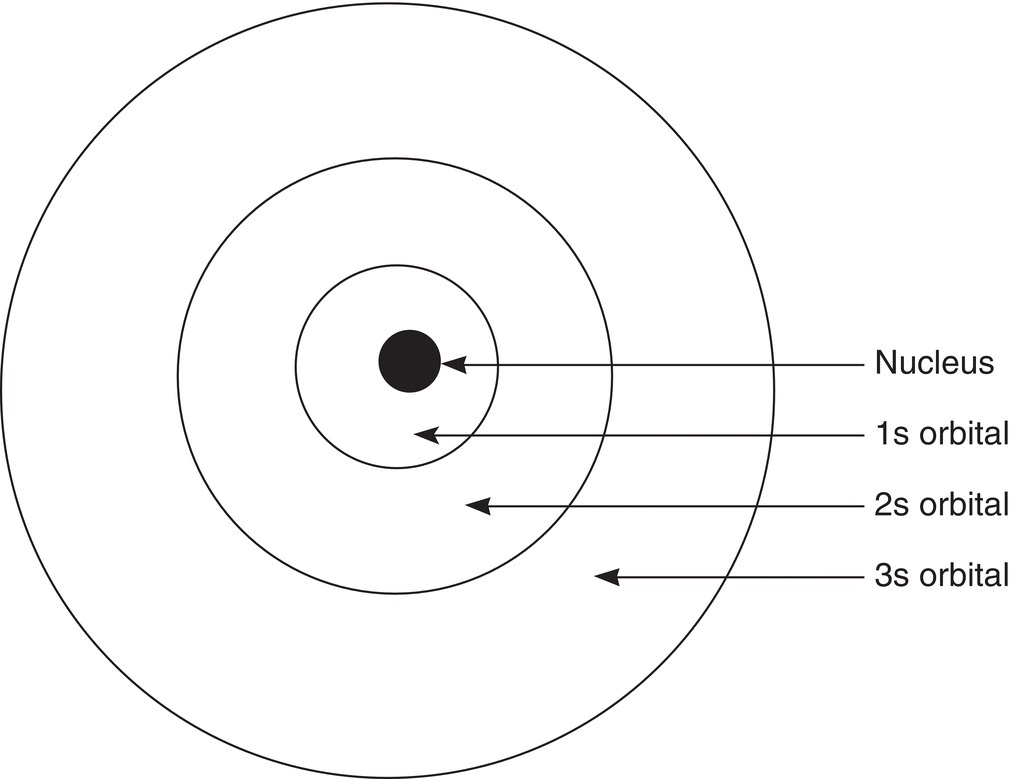
Figure 1.1 A slice through an atom showing s orbitals with different principal quantum numbers and different sizes. Remember that this is a two-dimensional representation of a three-dimensional atom; this is actually a slice through a sphere.
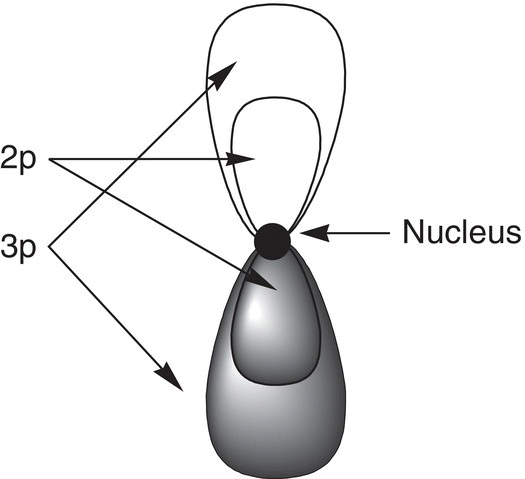
Figure 1.2 A slice through an atom showing p orbitals with different principal quantum numbers and different sizes. Remember that this is a two-dimensional representation of a three-dimensional atom.
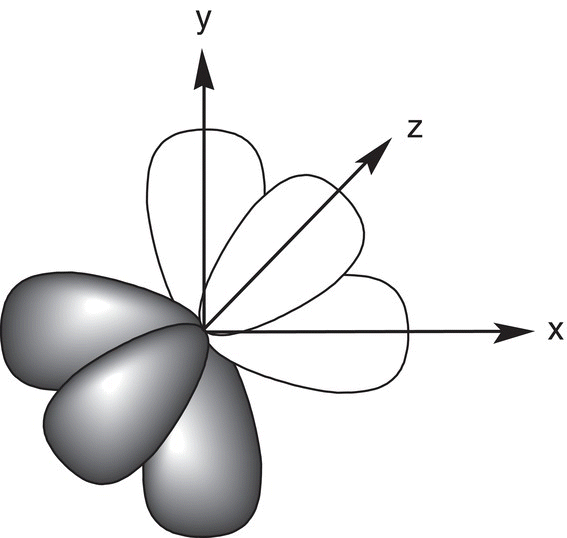
Figure 1.3 The three equivalent p orbitals all point in three different directions based on the x, y, and z planes.
In reality, the representation shown in Figure 1.2 for different p orbitals is only a partial description of p orbitals. For each p orbital that has the same principal quantum number, there are actually three equivalent p orbitals. All three equivalent p orbitals have exactly the same size and shape, but they are arranged in three different directions in the three-dimensional space. To differentiate between the three equivalent (or degenerate) orbitals, the subscripts, x, y, and z are used to indicate the direction in which they point in space. That is, for the 2p orbital shown in Figure 1.2, there are a total of three equivalent 2p orbitals as shown in Figure 1.3, 2px, 2py, and 2pz, and they all point in three different directions in the three-dimensional space. Similarly, for the 3p orbitals, there are a total of three 3p orbitals: 3px, 3py, and 3pz p orbitals.
Problem 1.1
List the following orbitals in order of their relative size:
2s, 2py, 1s, 2pz, 3s
In summary, Figure 1.4 gives an illustration of the orbitals around the nucleus of an atom.
1.2.2 Electronic Configuration of Atoms
Now that we have a good visual of the three-dimensional structure of the atom, we need to now concentrate on populating the atom with neutrons, protons, and electrons. As mentioned earlier, the nucleus has the neutrons and protons, and the electrons of an atom are located in regions outside the nucleus and they are not just randomly distributed, but they are in the different orbitals as described in the previous section. Let us start by looking at the simplest atom, the hydrogen atom. Try to locate this atom on the periodic table and identify the numbers associated with this atom. There are essentially two numbers associated with each atom on the periodic table as shown in Figure 1.5 for the hydrogen atom.
The first number is 1 (an integer and no units), which is the atomic number and indicates that there is only one electron and hence one proton. The other number is 1.0079 amu (atomic mass unit), which gives the average atomic mass of the atom based on the natural abundance of the different isotopes of the hydrogen atom. You will recall from your general chemistry course that isotopes have different number of neutrons. Based on our description of the orbitals, the one electron of hydrogen will be found in the orbital that is lowest in energy and closest to the nucleus. This orbital would be the 1s orbital, and not the 2s or the 2p orbitals, which are further from the nucleus and higher in energy.
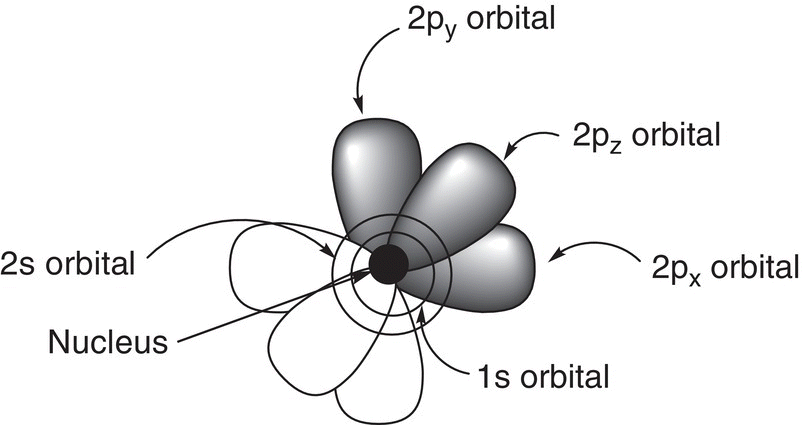
Figure 1.4 Two-dimensional illustration of the atom showing the nucleus, the 1s, 2s, 2px, 2py, and 2pz orbitals of an atom.
Let us examine the next and most encountered atom in organic chemistry, the carbon atom. Locate this atom on the periodic table and note its location relative to other atoms. Also note the different numbers that are associated with carbon, which are shown in Figure 1.6.
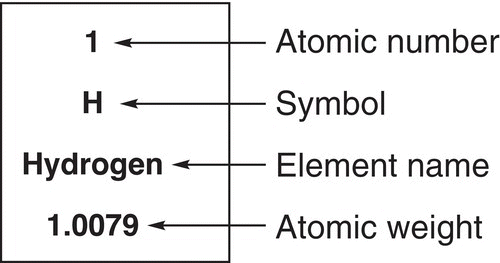
Figure 1.5 The hydrogen atom as seen on the periodic table.
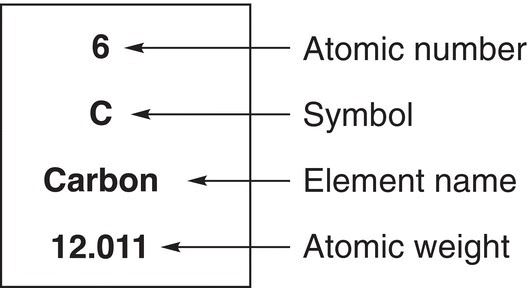
Figure 1.6 The carbon atom as seen on the periodic table.
You will notice the integer 6 (which indicates the number of electrons and hence the number of protons); there is the other number, 12.011, which is the atomic weight and represents the average mass of the isotopes of carbon that exist in natural abundance. Like that of the hydrogen atom, the number that is of most importance to us is the integer, which indicates the number of electrons that are present in the atom. Thus, based on the information obtained from the periodic table, a carbon atom has six electrons, but where are these six electrons? Are they all in the 1s orbital or are they all in the 2s, or 2p orbitals, or are they distributed randomly in these orbitals? Getting back to our original comparison of students populating the dorm, students are not randomly distributed in the various compartments of the dorm, but there are rules that must be followed to assign students to the different rooms. As mentioned earlier for the hydrogen atom, electrons much prefer to be closer to the nucleus since it is positively charged due to the presence of the protons. Thus, the electrons always occupy orbitals lower in energy (closer to the nucleus) first before occupying orbitals higher in energy; you will recall that this observation was described in your general chemistry course as the Aufbau Principle. Much like when students move into the dorm, they occupy the first floor first, and when that floor is full, then they start occupying the second floor, and so on. You will also recall from your general chemistry course that a maximum of two electrons can occupy any one orbital and the electron spin must be paired (Pauli’s Exclusion Principle). This principle is comparable to a rule for occupying the dormitory rooms, only two students to a room. Thus, of the six electrons of a carbon atom, only a maximum of two electrons can occupy any one orbital. Thus, the 1s orbital will have two electrons and the remaining electrons must occupy the other orbitals. The magnitude of the principal quantum number associated with an orbital indicates the relative energy of that orbital. Thus, the orbital next in energy to the 1s orbital is the 2s orbital. In order to determine the next set of orbitals, we will have to look at the principal quantum number. There are three 2p orbitals, and as a result, these orbitals are next in energy since they have the same principal quantum number. The next set of orbitals would then be the 3s, followed by the 3p orbitals. For the s and p orbitals with the same principal quantum number, the s orbital is lower in energy compared to the p orbital. Even though they both have the same principal quantum number, the s orbital is more compact, compared to the more diffused p orbital. Thus, for a carbon atom, two electrons would be in the 1s orbital, two would be in the 2s orbital, and the rest would occupy the 2p orbitals and not the 3s orbital.
As you will note from Figure 1.4, it is very cumbersome to utilize a two-dimensional artistic representation to effectively illustrate all the features of the atom, including the relative shapes, directions, and energies of orbitals. This representation is complicated even more when we try to show the electrons in the orbitals. A much easier representation that is often used to show the orbitals and the electrons that are contained in an atom is called the electronic configuration. With this representation, numbers, letters, subscripts, and superscripts are used to represent the relative energy (principal quantum number), shape, orientation, and number of electrons in each orbital, respectively. A superscript is used to represent the number of electrons in each orbital and a subscript is used to represent the orientation of each orbital in space. Subscripts are used in association with the p orbitals and not the s orbital since the s orbitals are spherical and not directional like the p orbitals. The electronic configurations for the atoms that will be encountered frequently in organic chemistry are shown below.
· Hydrogen (H): 1s12s02px02py02pz0 3s0 (or just: 1s1)
· Carbon (C): 1s22s22px12py12pz0 3s0 (or just: 1s22s22px12py1)
· Nitrogen (N): 1s22s22px12py12pz13s0 (or just: 1s22s22px12py12pz1)
· Oxygen (O): 1s22s22px22py12pz13s0 (or just: 1s22s22px22py12pz1)
Note that for orbitals of equal energy (degenerate orbitals), such as the 2px, 2py, and 2pz, electrons occupy separate orbitals unless there are more than one electron in each orbital; you will recall this observation as Hund's rule from your general chemistry course.
Problem 1.2
With the aid of your periodic table, give the electronic configuration for each of the following atoms: F, B, and Na.
1.2.3 Lewis Dot Structures of Atoms
Compounds are made of atoms held together by different bonds, which are formed by the electrons of the atoms. For these bonds, not all the electrons are involved, but typically electrons furthest from the nucleus and in the orbitals of highest energies. These electrons are classified into a category described as the valence electrons. By definition, valence electrons are the electrons of the outer shell of the atom, or electrons that are in orbitals with the same and highest principal quantum number. For example, there are four valence electrons for carbon, those are the electrons of the 2s and 2p orbitals; the two electrons that are in the 1s orbital are known as the core electrons. Note that even though there are two different types of orbitals, the s and the p, the principal quantum number for both is the same, which is 2 and hence classified as the valence shell. It is possible to determine the valence electrons for each atom from the periodic table, based on the number above the column of a particular atom. For our study in organic chemistry, the number of valence electrons will be of extreme importance when we examine bonding and reactions.
An American chemist, Gilbert N. Lewis (1875—1946) was the first to devise a method to easily show the number of valence electrons associated with different atoms by using dots; hence, these structures are known as the Lewis dot structures. In the Lewis dot structure, dots are arranged around an atom and the number of dots reflects the number of valence electrons in the atom as shown in Figure 1.7.
Note that for the atoms shown in Figure 1.7, only the valence electrons as shown in the square brackets are used for the Lewis dot structure.
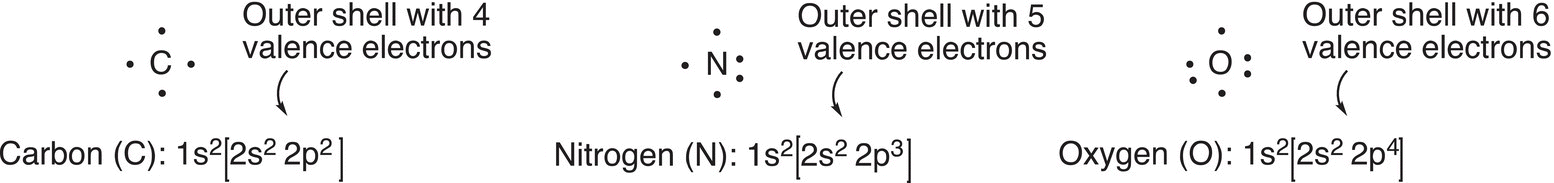
Figure 1.7 Lewis dot structures for selected atoms.
Problem 1.3
With the aid of your periodic table, give the electronic configuration and Lewis dot structures for the following atoms:
Li, Be, F, Ne, Na, and Mg.