Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Alkanes, Cycloalkanes, and Alkenes: Isomers, Conformations, and Stabilities
4.2 Structural Isomers
The simplest alkane that has structural isomers is butane since it is possible to have different bonding arrangements of the atoms. For butane, there are two different possible arrangements of the atoms that result in structurally different molecules with the same C4H10 formula as shown below.
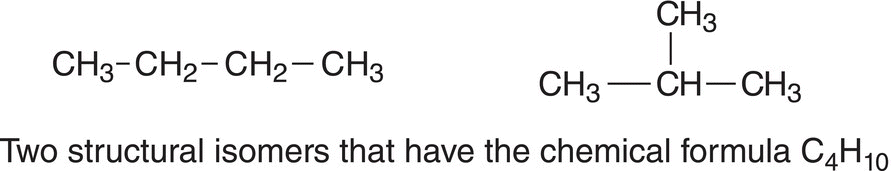
As mentioned earlier, the number of structural isomers increases as the number of atoms in a molecule increases. Molecules with many atoms have many more structural isomers, compared to molecules with a fewer number of atoms. Structural isomers are different molecules since the bonding arrangements are different for the compounds. The two compounds shown above with molecular formula C4H10 are known as structural isomers or constitutional isomers. Since structural isomers are different compounds, they have different properties, such as density and melting points.
Problem 4.1
i. Give structural formulas for all possible structural isomers of C5H12.
ii. Give IUPAC names for the isomers of question (i) above.