Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Bonding and Structure of Organic Compounds
1.6 Bonding — Concept Summary and Applications
Table 1.1 gives a summary of the differences between bonds formed to different hybridized orbitals of carbon.
An observation that can be made from Table 1.1 is that the carbon—carbon triple bond is the shortest bond of the three types of bonds involving hybridized orbitals. Recall that the carbon of the carbon—carbon triple bond uses sp orbitals and that they have 50% s character and 50% p character, compared to 25% s and 75% p for the carbon of methane. Since the s orbitals are closer to the nucleus, which is positive due to the presence of the protons, the attractions of the bonding electrons in the sp orbitals are stronger than those of methane, which contains only 25% s character. The carbon—carbon triple bond is also the strongest bond since its breakage requires the most energy (200 kJ mol−1), compared to methane or ethane.
Table 1.2 gives a more detailed summary of the bonding features of carbon to other common heteroatoms typically found in most organic chemistry. First, note the number of bonds that are possible to each atom. With carbon, even though there are different possible hybridization states, there are still only four bonds possible to each carbon. For nitrogen, even though it may have the same hybridization state as carbon, the number of bonds involving neutral nitrogen is different; there will always be three bonds to nitrogen if the formal charge on the nitrogen is zero. Likewise, oxygen, which has four hybridized orbitals, has just two bonds to the oxygen atom if the formal charge on oxygen is zero.
Table 1.1 Comparisons of the three types of orbitals found in most organic compounds.
Hybrid orbitals |
Angle around carbon |
Geometry |
Example |
C─C bond strength (kJ mol−1) |
C─H bond length (Å) |
C─C bond length (Å) |
sp3 |
109.5o |
Tetrahedral |
Ethane (CH3CH3) |
90 |
1.09 |
1.54 |
sp2 |
120o |
Trigonal planar |
Ethene (CH2═CH2) |
146 |
1.08 |
1.33 |
sp |
180o |
Linear |
Ethyne (H:C:::C:H) |
200 |
1.06 |
1.20 |
Table 1.2 Number of bonds that are possible from a neutral atom in molecules.
Atom |
Hybridized orbitals |
Number of bonds |
Geometry |
Bonds to other atoms |
Example |
Specific example |
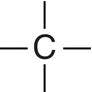
C |
sp3 |
4 |
Tetrahedral |
4 |
|
|
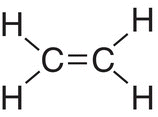
C |
sp2 |
4 |
Trigonal planar |
3 |
|
|
C |
sp |
4 |
Linear |
2 |
|
|
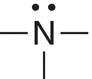
N |
sp3 |
3 |
Trigonal Pyramidal |
3 |
|
|
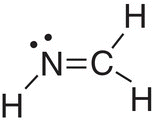
N |
sp2 |
3 |
Bent |
2 |
|
|
N |
sp |
3 |
— |
1 |
|
|
O |
sp3 |
2 |
Bent |
2 |
|
|
O |
sp2 |
2 |
— |
1 |
|
|