Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Addition Reactions Involving Alkenes and Alkynes
8.7 Addition of Carbenes to Alkenes
So far, the different reactive intermediates that have been discussed include radicals and carbocations. Another type of reactive intermediate is the carbene. Carbenes were discovered in 1959, they are neutral carbon species and have two bonds to carbon, instead of the typical four, which is expected for neutral carbon molecules. It was also observed that the most stable carbene contained a pair of electrons in a single orbital. In order to explain these observations, a systematic consistent scientific explanation must be developed to account for the structure of carbenes.
8.7.1 Structure of Carbenes
It was observed that there are two types of carbenes: one is described as a singlet carbene and the other as a triplet carbene. It is obvious that either of the traditional sp3, sp2, and sp hybridized orbitals could not provide an adequate description for this newly found species. The model that best explains these experimental results is one in which the carbon is sp2 hybridized, but the gap between the p and the three sp2 orbitals is larger than that found in the sp2 hybridized orbitals and p orbitals of the carbons for alkenes. That is, ΔG between the three equivalent sp2 orbitals and the p orbital is large and the electrons prefer to pair instead of going in separate orbitals. This type of carbene is called a singlet carbene (Figure 8.6).
8.7.2 Reactions of Carbenes
When considering the reaction of the very reactive carbene intermediate, there are two aspects that are very important: first, it has two electrons in a sp2 sigma (σ) orbital, which makes it nucleophilic. The second aspect is that it has an empty p orbital, which means that it is also electrophilic. Thus, it is possible for carbenes to have a very favorable reaction with the nucleophilic double bond to produce an electrophilic carbon on the alkene, which can then react with the nucleophilic two electrons in the sigma (σ) orbital of the carbene to produce a neutral product as shown in Reaction (8-81) to produce a cyclopropane ring. As a result, this type of reaction is often referred to as cyclopropanation of alkenes and is a concerted reaction and does not occur in a step-wise manner as described for some of the other reactions studied.
σσ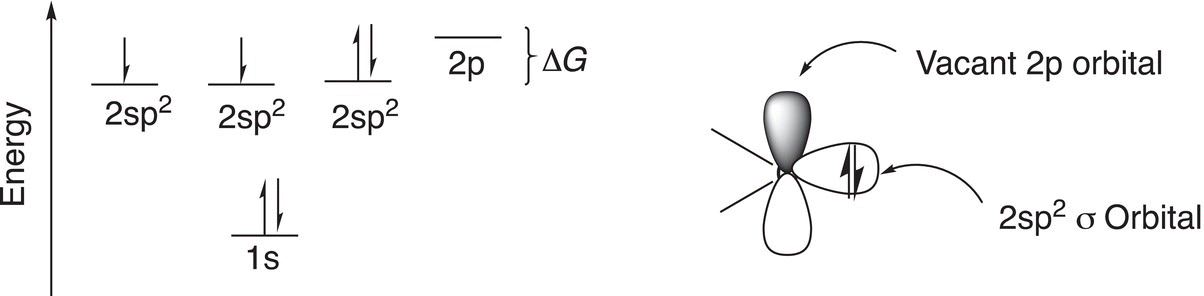
Figure 8.6 Proposed model for the electronic configuration of singlet carbenes.
(8-81)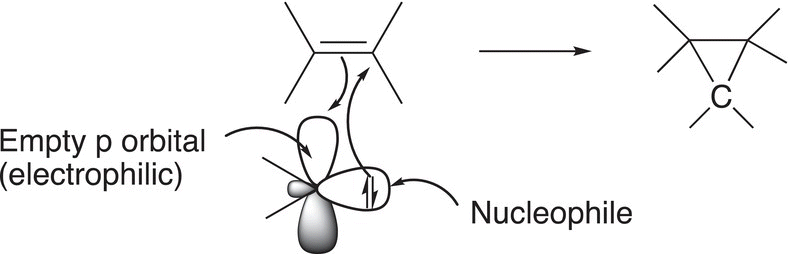
The Simmons—Smith reaction is commonly used to generate carbenes for the cyclopropanation of alkenes. The main reactant intermediate of the Simmons—Smith reaction is a “carbinoid” species since a carbene is not actually formed, as illustrated in Reaction (8-82).
(8-82)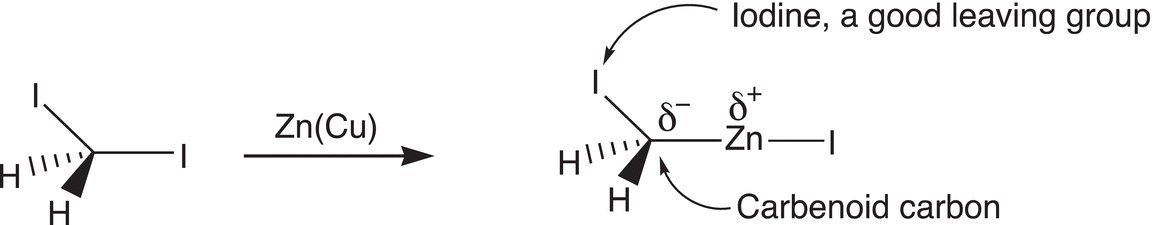
The initial product of the Simmons—Smith reaction is classified as carbenoid, and not a carbene. This species contains a very good leaving group, iodine that is bonded to a carbon. The same carbon is also bonded to a zinc, which means that the bonding electrons of the polar carbon—zinc bond are closer to the more electronegative carbon than zinc, hence the carbon of this compound is classified as carbene-like hence, “carbenoid.” Reaction (8-83) gives an example of cyclopropanation using the Simmons—Smith reaction.
(8-83)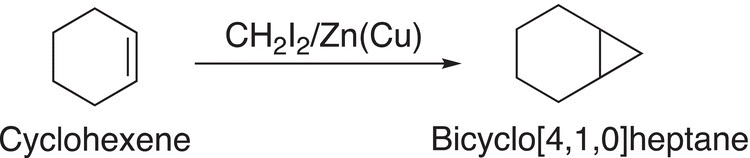
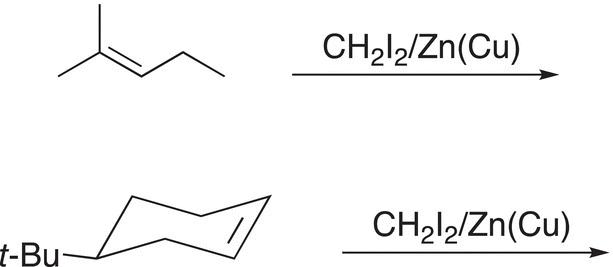
Problem 8.13
Give the major organic product of the reactions shown below.
Another method for the generation of carbenes is accomplished by exposing diazomethane to energy in the form of heat or light, as shown in Reaction (8-84).
(8-84)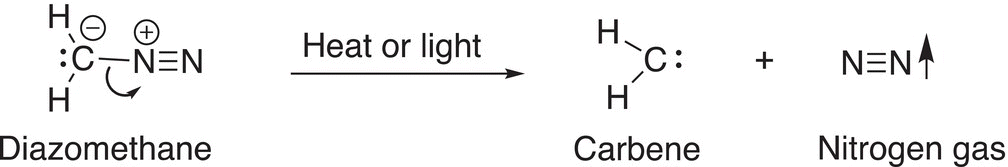
Diazomethane is very reactive since it liberates a gas (nitrogen) and hence an increase in entropy when exposed to energy to break the C─N bond to produce a carbene and nitrogen.