Physical Chemistry: A Very Short Introduction (2014)
Chapter 7. Investigating matter
Physical chemistry lies at the heart of one of chemistry’s principal applications and achievements: the identification of the substances present in a sample and the determination of their abundances and structures. As these techniques increase in sophistication, physical chemists are ever more deeply involved in the interpretation of the rich data that they provide. They make use of many of the concepts that I have introduced in the preceding chapters, contributing through quantum theory (in spectroscopy), thermodynamics (through thermochemistry and electrochemistry), and kinetics. In many cases, a small army of concepts is responsible for the technique and its interpretation.
Two items have contributed enormously and almost universally to the elaboration of classical techniques: the laser and the computer. The laser has refined spectroscopy and kinetics, and has played a role even in thermochemistry. Few pieces of apparatus beyond the simplest are not touched either for control or interpretation by a computer, and the computer itself has enabled the entire field of computational chemistry.
Spectroscopy
Light, and electromagnetic radiation in general, brings information from the innermost parts of atoms and molecules. The detection and analysis of the wavelengths and frequencies present in that light has been the source of an enormous amount of information and the techniques based on it continue to be developed. There are four flavours of spectroscopy—emission, absorption, scattering, and resonance—and different techniques for the different regions of the electromagnetic spectrum.
In emission spectroscopy, which is largely confined to the identification and study of atoms, light is emitted from atoms that have been energetically excited and then emit a photon as they collapse back to states of lower energy. The yellow light emitted by sodium atoms in street lighting is an example. As well as identifying the presence of elements in a sample, this kind of atomic spectroscopy gives very detailed information about the internal structures of atoms and was the original stimulus of the application of quantum mechanics to atoms.
Absorption spectroscopy is mainstream spectroscopy. In it, a beam of radiation of variable but precisely known frequency is passed through a sample and the attenuation of its intensity is recorded. There are three main sub-varieties. Microwave spectroscopy monitors the absorption of microwave radiation (radiation of wavelengths of several centimetres) by molecules that are free to rotate in the gas phase. It relies on the excitation of rotations and provides very precise information about bond lengths and bond angles. Infrared spectroscopy makes use of the absorption of infrared radiation by the vibrations of molecules and is used to identify the molecule from its vibrational fingerprint. Ultraviolet/visible spectroscopy (‘UV/vis spectroscopy’) similarly monitors absorption, but now towards shorter wavelenths and higher frequencies. Absorption at these frequencies is due to the excitation of electrons out of the orbitals they occupy in the ground state. The sudden redistribution of electron density stimulates the molecule to burst into vibration and that bursting into vibration can also cause the molecule to rotate at different rates. The consequences of the stimulation of vibration and rotation when an electron is excited in a molecule can be detected in the gas phase and used to extract information about the strengths and stiffness of bonds; in solution they blur the absorption and give rise to broad bands in the spectrum.
I referred to scattering as one of the flavours. This is the domain of Raman spectroscopy, which was invented by Chandrasekhara Raman (1888–1970) and Kariamanickam Krishnan (1898–1961) in 1928 and for which Raman but not Krishnan quickly received a Nobel Prize (for physics) in 1930. The ‘Raman effect’ is the inelastic scattering of light by molecules. In the technique, photons of light (and ultraviolet radiation) are directed on to the sample and are scattered by its molecules. The scattered light has either a lower frequency if in its interaction with the molecules it loses energy to them or it has a higher frequency if it gains energy during the collision. Examination of the frequencies present in the scattered light reveals what energies the molecules can have and from that information can be deduced information about their identity and structure. Raman spectroscopy was little more than a laboratory backwater until the development of lasers because the intensity of the scattered radiation is low and concealed under the incident radiation unless the latter covers a very narrow band. Lasers provide intense, almost single-frequency (‘monochromatic’) light, so both these problems are overcome and Raman spectroscopy has moved into the mainstream of spectroscopic techniques. Allied with microscopy, it is also a powerful technique for the detailed analysis of surfaces.
The fourth flavour, resonance, is so important that I give it its own section.
Magnetic resonance
We all use resonance every day. Resonance occurs when the natural frequency of a device is tuned to the same frequency as a stimulus, such as the electromagnetic radiation from a distant radio transmitter. The response of the device is greatest when it is so tuned, and all kinds of radio communication depend on it. In its simplest application in spectroscopy, the properties of molecules are modified by controlling their environment (in all the applications I shall describe, by changing the strength of a magnetic field) until they are brought into resonance with the frequency of an oscillating electromagnetic field.
The hugely important technique of nuclear magnetic resonance (NMR), which was invented by physicists in the 1940s but quickly adopted by chemists to the point that no self-respecting laboratory can be without it, is a radiofrequency technique that uses (in modern applications, superconducting) magnets to influence the molecules it investigates. Many people have been the sample in an NMR observation, for it is used as a diagnostic technique as magnetic resonance imaging (MRI), where the ‘nuclear’ has been deleted from the name so as not to frighten the squeamish.
The ‘nuclear’ in NMR has nothing to do with nuclear radiation, only with nuclei. I mentioned in Chapter 1 that electrons have the property evocatively named ‘spin’. Many nuclei have the same property: a hydrogen nucleus, the proton, has spin; a carbon nucleus (specifically the common isotope carbon-12) does not. A charged spinning body behaves like a bar magnet, so a nucleus with spin does so too. The north–south direction of the magnet depends on the direction of its spin, which in the case of a proton (like an electron) can be either clockwise or counterclockwise. It follows that protons (and nuclei with similar spin) will have different energies in an applied magnetic field depending on whether the bar magnet is north-up or north-down, and the energy separation will depend on the strength of the applied field (Figure 24). It is the ability to vary that energy separation that constitutes the ‘tuning’ in NMR. For the typical magnetic fields used in modern NMR, electromagnetic radiation in the region of 500 MHz is common, which lies in the ‘radiofrequency’ region of the spectrum (a little above FM radio which is close to 100 MHz).
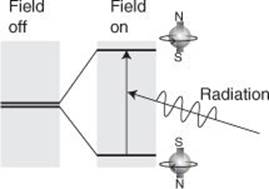
24. The basic process in NMR is to tune the separation of the two energy levels of a proton in a magnetic field to the frequency of a radiofrequency field. At resonance, the incident radiation is absorbed
The technique would be of little consequence in chemistry if all it achieved was flipping a spinning proton from one orientation to another. It acquires its extraordinary usefulness from two principal features (and in MRI a third). One feature is that protons in different parts of the same molecule experience slightly different magnetic fields from that applied: the applied field stirs up local currents in the molecules that augment or diminish the field, so resonances occur at different applied field strengths. These different ‘chemical shifts’ give valuable information about what groups of atoms are present. Second, a proton responds to the magnetic field generated by protons a bond or two away, and the interaction splits the single resonance absorption into characteristic patterns. This ‘fine structure’ helps to identify the molecule.
Modern NMR is far more sophisticated than this elementary account suggests, and physical chemists have contributed significantly to its development. Most NMR spectrometers use pulses of radiofrequency radiation to twist the spins of groups of protons into new orientations and to observe how protons twisted into high-energy orientations fall back into lower energy orientations. These are the so-called Fourier-transform NMR (FT-NMR) spectrometers, which use mathematical procedures to extract spectra from the data obtained from the sequence of pulses.
Clever pulse sequences, the replacement of non-magnetic carbon-12 nuclei by magnetic carbon-13 nuclei, extending the range of spectrometers to observe the resonance of phosphorus and fluorine nuclei, and inserting magnetic ions into the structure in known locations are all used not only to identify complex molecules but also to establish their structures. This application augments the information from X-ray diffraction (Chapter 4) because structures can be determined in natural aqueous environments typical of the interiors of biological cells, which might differ significantly from the structures adopted when the molecules lie artificially imprisoned together in crystals. Further developments in NMR technology are opening up the possibility of performing observations on samples of nanometre scales.
Nuclear magnetic resonance is also used to identify the motion of molecules in liquids and to establish the dynamical properties of the membranes that form the walls of biological cells. There are even hints that NMR might form one technique for realizing a quantum computer; so there might come a day when an NMR spectrometer understands what it is doing. The version of NMR used in MRI scans enables physicians to examine soft tissue non-invasively: it depends on exposing organisms to carefully controlled magnetic and radiofrequency electromagnetic fields, detecting the relaxation times of protons in different environments, and reconstructing three-dimensional images of the distribution of protons.
I called this section ‘magnetic resonance’ not ‘nuclear magnetic resonance’ because there are other magnetic resonance spectroscopies. Thus, electron paramagnetic resonance (EPR; or electron spin resonance, ESR) is similar to NMR but makes use of the magnetic properties of electrons. It is confined to molecules that have an unpaired electron (these include the radicals I mentioned in Chapter 6), so is far less widely applicable than NMR. However, it gives valuable information on these species and on some types of biological molecules (haemoglobin, for instance).
Mass spectrometries
A ‘mass spectrum’ is a totally different animal from the spectra I have mentioned so far, for the spectrometer used to obtain it detects fragments of molecules that are distinguished by their different masses. The principle involved is straightforward: smash a molecule into pieces, accelerate the ions to a speed that depends on their mass, and use a device that detects these different masses by deflecting them with an electric or magnetic field (the deflection also depends on the mass and speed) and recording the fragments that make the journey to a detector. The task before the experimenter is then to infer the identity and structure of the original molecule from the collection of fragments.
Mass spectrometry is widely used in organic chemistry to help identify compounds. One limitation that restricted its application to the large molecules of such importance in biology is the difficulty of getting enough of them present as a vapour so that the fragmentation of them by the impact of an electron beam was viable. That difficulty has largely been solved by embedding them in a polymeric material and then blasting it and the molecules into space by vaporizing it with a laser beam.
I called this section ‘spectrometries’ in the plural because I wanted to sneak in a mention of a variation of mass spectrometry that is more central to physical chemistry than is conventional mass spectrometry. Photoelectron spectroscopy (PES) is used both to explore the energies with which electrons are bound inside molecules and to identify species on surfaces.
In the technique, molecules are exposed to ultraviolet radiation that causes the ejection of electrons; these electrons replace the molecular fragments of conventional mass spectrometry, and otherwise the procedure is the same. The ejected electrons are accelerated and focused on a detector. By varying the strength of the field used to deflect them, the energy with which they left the molecule can be inferred. That energy is the difference between the energy of the photon of radiation that ejected them and the strength with which they were bound, so the latter can be deduced. This type of observation augments the calculations of computational chemistry and acts as an experimental verification of the energies that are computed as well as building up a more complete picture of the electronic structure of the molecule than ultraviolet spectroscopy can provide alone.
In a modification with the pedestrian name electron spectroscopy for chemical analysis (ESCA) the ultraviolet radiation of conventional PES is replaced by more energetic X-rays. Photons of X-rays are so energetic that they can eject the electrons that lie very close to nuclei. These electrons don’t take part very much in chemical bonding so they have almost the same energy as in the unbound atom, and therefore the detected energies are characteristic of the element regardless of its state of combination and therefore can be used to identify the matter present.
Surface studies
Surfaces, despite being the outward show of a solid, have proved very difficult to study yet are of great importance, especially in catalysis. As I remarked in Chapter 6, catalytic action, the acceleration of chemical reactions, takes place on them. As I also mentioned there, their study was transformed some years ago by the introduction of a new technique and its elaborations.
The transformative technique is scanning tunnelling microscopy (STM). The key word here is ‘tunnelling’, which is a term borrowed from quantum mechanics and denotes going where classical mechanics forbids. The technique, as perhaps is true of most techniques that rely so centrally on quantum mechanics, is really most extraordinary and perhaps unlikely. In it, a metal probe is pulled out to form a very fine point, which is then dragged in a series of straight lines and at constant height across the surface of the sample (there are variations in this procedure, but I will focus on this one); this is the ‘scanning’ part of the name. At the same time, the electric current passing through the tip is monitored and its variation used to map the rise and fall of the surface (Figure 25). The key point is that it is classically forbidden for electrons to jump across the gap between the tip of the probe and the surface, but quantum mechanics allow the electrons to cross the gap (this is the ‘tunnelling’ part of the name). This tunnelling is extremely sensitive to the width of the gap; it is so sensitive that it is sensitive to the rise and fall of the surface on an atomic scale, and the portrayal of the surface that results shows the individual atoms of the surface lying in serried ranks, with chasms and cliffs atoms deep and high, and reveals the shapes of individual molecules attached to the surface (this is the ‘microscopy’ of the name). Atoms, through STM, have at last become visible (Figure 26).
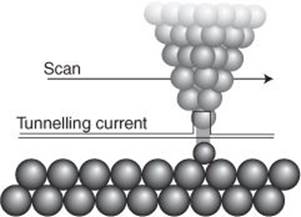
25. The process occurring at the tip of a scanning tunnelling microscope: the flow of current across the gap is highly sensitive to the width of the gap, and even atom-scale variation can be detected
A variation of STM turns the surface chemist from passive observer into active participant. In atomic force microscopy (AFM) the tiny probe is used to nudge atoms around on the surface and in effect to build individual molecules atom by atom.
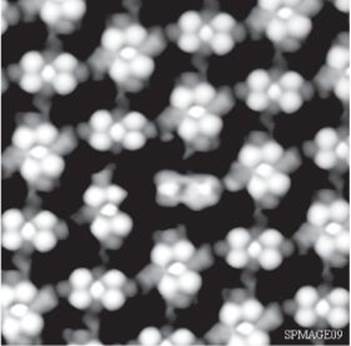
26. A typical STM scan, showing individual atoms on a surface
Surface chemists, the physical chemists who study surfaces, still continue to use classical techniques to study surfaces because the arrangement of atoms is not the only kind of information they need. A crucial part of the understanding that a surface plays in the promotion of chemical reactions is how much reactant adsorbs on the surface and its nature when it is there. Physical chemists have built models of the processes taking place on surfaces, have found relations between the amount adsorbed and the pressure of the overlying material in the gas, and have studied the energetics of adsorption. They find it initially helpful to distinguish between ‘physisorption’, when the molecule attaches unchanged to the surface, and ‘chemisorption’, when the adsorption occurs with the breaking and formation of covalent bonds and in some cases is accompanied by the fragmentation of the molecule. Chemisorption is the key to catalysis, for a molecule at least partially torn apart is ripe for reaction.
The surface area exposed by a catalyst is important for its function, for it is normally the case that the greater that area, the more effective is the catalyst. Surface chemists have methods for determining the area and of making it greater. One procedure is to synthesize so-called ‘microporous’ materials which (as mentioned in Chapter 4) are solids so riddled with pores, chambers, and channels that they are effectively all surface, with a gram of material having the surface area the size of a tennis court. Graphene, the material that has caused so much excitement on account of its unique mechanical, optical, and electrical properties and which has already won a Nobel Prize (for the physicists Andre Geim and Konstantin Novosolev in 2010) is essentially a single layer of chickenwire-like hexagonally arranged carbon atoms, as in the solid graphite. It is almost pure surface, and currently the target of the exploration of much surface chemistry.
Lasers
Lasers have transformed many aspects of physical chemistry and are common features in laboratories. Three of the characteristics of the radiation they produce are responsible, and I have touched on them in Chapter 6.
One is their intensity. Each pulse of radiation can be regarded as a hurtling swarm of an enormous number of photons. This feature rejuvenated Raman spectroscopy for even a small proportion of inelastically scattered photons from that swarm can still give rise to a reasonably intense beam to detect, record, and analyse. Intensity also brings into range so-called ‘non-linear optical phenomena’, in which more than one photon might interact simultaneously with a molecule instead of just the normal one-photon/one-molecule interaction, and give rise to new phenomena and new ways to explore its structure. The new phenomena include the separation of isotopes by laser irradiation and new opportunities for using laser radiation in chemical synthesis. Even the light that is scattered from molecules without change of frequency, the so-called ‘Rayleigh scattering’, can be employed to assess the sizes of the large molecules typical of polymers and to examine the motion of molecules in fluids.
Physical chemists also make use of the monochromaticity of laser radiation, the fact that it consists of a very narrow range of wavelengths and frequencies. Not only does that narrowness make Raman spectroscopy more feasible than when it had to rely on conventional optical sources but it also gives the experimenter great precision in selecting the state to which molecules are excited in a photochemical experiment.
I also explained in Chapter 6 how the sharpness of the pulses from lasers can be employed to investigate processes that occur very rapidly. A process can be stimulated with a short, sharp pulse, and then examined almost instantly later by sending in another pulse and monitoring its absorption. Thus investigations of processes taking place in fractions of a second (even attoseconds, 1 as = 10–18 s) can be made and extraordinarily detailed pictures of chemical reactions constructed.
The intense field of a laser beam has another, entirely different application: when focused down to almost a point, it can act as ‘optical tweezers’ and hold tiny particles of matter in place for them to be studied individually. Other spectroscopic techniques are also emerging which enable single molecules to be studied. This type of study is especially important for watching the possibly complex behaviour of biological molecules as they participate in biochemical processes.
Computers
Computers have profoundly affected the instrumentation in modern physical chemistry laboratories and have applications well outside their intrinsic contribution to computational chemistry, the quantum mechanical calculation of molecular structure, including the still vexed question of how the long amino acid chains of protein molecules fold into the precise, semi-rigid shapes that are crucial to their function. The latter falls, to some extent, into the field of ‘molecular mechanics’ where Newton’s laws of classical physics are used (the quantum mechanics being plain too difficult) to predict how a molecule or parts of a big molecule move under the influence of the forces acting within the molecule. Physical chemists have identified most sources of these forces. The difficulty with coping with them is that some are long-range, acting over the width of a molecule, some are short-range, acting only when parts of molecules come into contact, and some aren’t really forces at all. By that last enigmatic remark I mean that any water around parts of a biological molecule can influence its motion as though it were exerting a force. Building all these effects into the model is very demanding and remains a focus of much current research.
Computers control spectrometers and diffractometers (for X-ray diffraction). Modern spectroscopic techniques commonly use indirect procedures for obtaining spectra, the data needing to be manipulated by quite extensive computation. The useful analogy that illustrates these ‘Fourier transform’ techniques is that of determining the frequencies (the notes) present in a piano. One way is to play each note in turn and to record its presence. An alternative is to drop the piano and record the awful noise as it, the noise, fades away. The individual notes that contribute to that noise can be identified by taking the recording of the noise and performing on it the same kind of mathematical transformation, the Fourier analysis. Such procedures, which are used in several branches of spectroscopy, including NMR, greatly accelerate the collection of the spectral data and provide highly detailed information.
The current challenge
Old techniques become more sophisticated and reveal information that was out of the range of their predecessors; new techniques open our intellectual eyes to new properties. Both bring in their train problems of interpretation and the elaboration of old models of Nature. Physical chemistry is a happy marriage of experiment and theory, the latter being inspired by the former and the theory inspiring new experiments. Often those experiments simply confirm what has been supposed or add quantitative spine to qualitative ideas. But often they open up whole new landscapes for physical chemistry to enter and explore.
The laser goes on producing surprises and opportunities, such as exploring ever shorter timescales and enabling physical chemists to examine single molecules to understand both their structures and their dynamical behaviour. Computing, especially high-performance computing, where vast computational resources can be brought to bear to generate simulations of elaborate systems, is undoubtedly the way forward and will bring opportunities for understanding that are beyond our, or at least my, imagining. Detailed information on an atomic scale is being obtained by using very intense radiation sources, such as the radiation generated by synchrotron sources (a national facility in many regions, as they are huge machines), including the extension of X-ray studies of proteins to their dynamical behaviour. Microscopy of various flavours is used in tandem with spectroscopies to extract detailed information about surface phenomena. Even new forms of matter, such as the special kind of matter, the so-called Bose condensate, that forms when miniscule collections of atoms are isolated and cooled to such low temperatures that quantum phenomena become dominant, are becoming open to investigation.
Not only do physical chemists contribute to the development of new techniques for the study of conventional, intriguing, novel, forms of matter, but they also contribute to the extraction of the information that these ever more sophisticated techniques provide. They are extending their domain of enquiry and elucidation to nanostructures, where entirely new kinds of instrumentation and theoretical analysis are needed, and increasingly to biology, where traditional phenomena collaborate, display complex behaviour, and cannot be understood without computer analysis and simulation. Physical chemists will never be left with nothing to do.