Chemistry: A Self-Teaching Guide - Post R., Snyder C., Houk C.C. 2020
Chemical Equations
Now that you are familiar with atoms, symbols, molecules, formulas, and nomenclature, let's look at what happens when we mix substances together. The most important result of your efforts with this chapter will be your ability to write a balanced chemical equation that represents the reaction between two or more different substances that produces at least one new substance.
Chemical equations are the chemist's shorthand. They show at a glance what substances have been mixed together and what new substance(s) have been produced. Chemists are able to predict the products of a mixture of substances even though they may never have actually mixed the substances in the laboratory. This is very important to research chemists trying to prepare new products that are useful and beneficial to mankind.
You will learn how to complete and balance several kinds of chemical equations and how chemists recognize whether or not a reaction does indeed occur when substances are mixed.
You will discover that some things remain unchanged during a chemical reaction. Chemists are more concerned with the things that change, so you will learn a scheme that shows what does change during certain chemical reactions.
OBJECTIVES
After completing this chapter, you will be able to
· recognize and apply or illustrate: reactant, product, electrolyte (weak or strong), precipitate, soluble, insoluble, solid, liquid, gas, and aqueous;
· balance a molecular equation when given the formulas of the reactants and products;
· convert word equations to balanced molecular equations;
· convert balanced molecular equations to complete ionic equations and then to net ionic equations;
· identify a chemical equation as an example of a combination, decomposition, single displacement, or double displacement type of reaction;
· explain why or how we recognize whether or not a chemical reaction occurs;
· use a solubility table to determine if a compound is soluble or insoluble.
![]() Chemical equations are useful to the chemist because they represent chemical reactions. You have already learned that certain chemical symbols represent molecules, elements, ions, or compounds. These symbols are also used in chemical equations. The substance or substances that are the starting materials in a chemical reaction are called reactants and are located on the left side of a chemical equation. The substance or substances produced as the result of a reaction are called products and are located on the right side of a chemical equation.
Chemical equations are useful to the chemist because they represent chemical reactions. You have already learned that certain chemical symbols represent molecules, elements, ions, or compounds. These symbols are also used in chemical equations. The substance or substances that are the starting materials in a chemical reaction are called reactants and are located on the left side of a chemical equation. The substance or substances produced as the result of a reaction are called products and are located on the right side of a chemical equation.
Hydrogen gas and oxygen gas react to produce water. The chemical equation for this reaction is 2H2 + O2 → 2H2O.
1. Identify the symbol(s) representing the reactant(s).
2. Identify the symbol(s) representing the product(s).
Answer: (a) H2 and O2; (b) H2O
![]() Some reactions require continuing energy in order to occur. Some reactions occur spontaneously when the reactants are mixed together. Other reactions just need energy to get started and will then continue until one or more reactant is used up.
Some reactions require continuing energy in order to occur. Some reactions occur spontaneously when the reactants are mixed together. Other reactions just need energy to get started and will then continue until one or more reactant is used up.
Later in this chapter we will deal with predicting whether or not chemical reactions will actually occur as written.
The following are examples of two types of chemical reactions. The equation 2H2 + O2 → 2H2O represents a combination reaction because two or more different reactants combine to form a single product. The equation 2H3PO4 → H4P2O7 + H2O represents a decomposition reaction because a single reactant is decomposed to form two or more different products.
1. The equation 2H2O2 → 2H2O + O2 represents what type of reaction? __________
2. Identify the reactant(s) and product(s) in the equation. __________
Answer: (a) decomposition reaction; (b) H2O2 is the reactant. It is on the left side of the equation. H2O and O2 are the products, on the right side of the equation.
![]() Identify each of the following equations as either decomposition or combination.
Identify each of the following equations as either decomposition or combination.
1. 2C + O2 → 2CO
2. 2HO → 2Hg + O2
3. H2 + Cl2 → 2HCl
Answer: (a) combination; (b) decomposition; (c) combination
![]() The following equation represents a single displacement reaction.
The following equation represents a single displacement reaction.
![]()
In the reaction, the metal magnesium (Mg) has displaced the hydrogen atom (H) in HCl and formed magnesium chloride and hydrogen gas (H2) as products. In a single displacement reaction, an element and a compound react to form another element and compound.
In contrast with the single displacement reaction, the following equation is called a double displacement reaction; two compounds react to form two different compounds.
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
In this reaction, the Ba displaces the Na in Na2SO4 to form BaSO4. The Na displaces Ba in BaCl2 to form NaCl. In the following examples, two substances react to form two different substances.
1. 2KCl + 2HgNO3 → 2KNO3 + Hg2Cl2
2. 2Na + H2SO4 → Na2SO4 + H2
1. What type of reaction does equation 1 represent?
2. What type of reaction does equation 2 represent?
Answer: (a) double displacement (involves two compounds reacting to form two different compounds); (b) single displacement (involves a compound and an element reacting to form a different compound and an element)
![]() Identify the following reaction equations as combination, decomposition, single displacement, or double displacement.
Identify the following reaction equations as combination, decomposition, single displacement, or double displacement.
1. 2KClO3 → 2KCl + 3O2______________________
2. H2 + Cl2 → 2HCl______________________
3. Zn + Cu(NO3)2 → Cu + Zn(NO3)2______________________
4. AgNO3 + KCl → AgCl + KNO3______________________
5. FeCl3 + 3KOH → Fe(OH)3 + 3KCl______________________
Answer: (a) decomposition; (b) combination; (c) single displacement; (d) double displacement; (e) double displacement
![]() Double displacement reactions are especially important because a large number of reactions are of this type. Think of double displacement reactions as compounds exchanging partners. Later in this chapter you will need to predict the products of double displacement reactions when given the reactants. In the following generalized reaction equation, A and B are positive ions (e.g., metal,
Double displacement reactions are especially important because a large number of reactions are of this type. Think of double displacement reactions as compounds exchanging partners. Later in this chapter you will need to predict the products of double displacement reactions when given the reactants. In the following generalized reaction equation, A and B are positive ions (e.g., metal, ![]() , or H+) and X and Y are negative ions (one or more nonmetal atoms of an ionic compound that remain after the positive ion is removed).
, or H+) and X and Y are negative ions (one or more nonmetal atoms of an ionic compound that remain after the positive ion is removed).
![]()
In this equation, A has displaced B and B has displaced A.
In a double displacement reaction, the metal, or ![]() , or H+ ion of one reactant replaces the metal, or
, or H+ ion of one reactant replaces the metal, or ![]() , or H+ ion in the other reactant, and vice versa.
, or H+ ion in the other reactant, and vice versa.
In the following double displacement reaction, the products can be predicted.
![]()
The positive metal ions are Ag+ and Na+. The negative ions are ![]() and Cl−. Predict the products of this reaction. __________
and Cl−. Predict the products of this reaction. __________
Answer: AgCI and NaNO3
![]() Predict the products of the following double displacement reactions.
Predict the products of the following double displacement reactions.
1. CuSO4 + Ca(OH)2 → __________________________
2. NaC2H3O2 + HCl → __________________________
Answer: (a) CaSO4 and Cu(OH)2; (b) NaCl and HC2H3O2
BALANCING CHEMICAL EQUATIONS
![]() In an actual reaction, all atoms must be accounted for. Atoms are neither gained nor lost. All of the equations thus far have been balanced: that is, the number of each type of atom on the reactant side of the equation is equal to the number of each type of atom on the product side. The balancing of chemical equations is very important to chemists, since only a balanced equation can adequately describe the ratios of reactants and products in a reaction. An equation that is not balanced does not truly represent a reaction. Look at the following chemical equation:
In an actual reaction, all atoms must be accounted for. Atoms are neither gained nor lost. All of the equations thus far have been balanced: that is, the number of each type of atom on the reactant side of the equation is equal to the number of each type of atom on the product side. The balancing of chemical equations is very important to chemists, since only a balanced equation can adequately describe the ratios of reactants and products in a reaction. An equation that is not balanced does not truly represent a reaction. Look at the following chemical equation:
![]()
1. How many atoms are on the reactant side? H _____, Cl _____
2. How many atoms are on the product side? H _____, Cl _____
3. Is the equation balanced? _____
Answer: (a) two, two; (b) one, one; (c) no (The atoms on the reactant side do not equal those on the product side.)
![]() Balancing equations is often a matter of trial and error. Coefficients (usually of small whole numbers) can be placed in front of reactants or products, when necessary, to obtain equal numbers of atoms on both sides of the equation. Do not change the subscripts within the formulas of the reactants or products.
Balancing equations is often a matter of trial and error. Coefficients (usually of small whole numbers) can be placed in front of reactants or products, when necessary, to obtain equal numbers of atoms on both sides of the equation. Do not change the subscripts within the formulas of the reactants or products.
The molecular formula for water is H2O. To double the formula for water, which of the following is the correct expression: H4O4, H4O2, 2H2O? _________________
Answer: 2H2O (The other two expressions have changed the actual molecular formula.)
![]() The expression 2H2O indicates two molecules of H2O. This results in twice as many hydrogen atoms (and doubles the oxygen atoms also). H2SO4 is the formula for sulfuric acid and contains two atoms of H, one atom of S, and four atoms of O. The expression 3H2SO4 indicates three molecules of H2SO4 containing _____ atoms of H, _____ atoms of S, and _____ atoms of O.
The expression 2H2O indicates two molecules of H2O. This results in twice as many hydrogen atoms (and doubles the oxygen atoms also). H2SO4 is the formula for sulfuric acid and contains two atoms of H, one atom of S, and four atoms of O. The expression 3H2SO4 indicates three molecules of H2SO4 containing _____ atoms of H, _____ atoms of S, and _____ atoms of O.
Answer: six; three; 12
![]() Let's consider what is meant by the word “equation.” The following mathematical expression is an equation because both sides are equal. It is “balanced” because each side is equal to 12.
Let's consider what is meant by the word “equation.” The following mathematical expression is an equation because both sides are equal. It is “balanced” because each side is equal to 12.
![]()
In the purest sense of the word, a chemical “equation” that is not balanced is not a true equation because the two sides are not equal. Only a balanced equation is a true mathematical equation or true chemical equation. To balance a chemical equation, you may place coefficients (smallest possible whole numbers) in front of any substance in the equation on a trial and error basis until the equation is balanced with equal numbers of atoms on each side.
Balance the following equation by placing coefficients in front of appropriate substances. The coefficients should be the smallest possible whole numbers.
![]()
Answer: H2 + Cl2 → 2HCl
Since the reactant side has two H atoms and two Cl atoms and the product side has only one H atom and one Cl atom, placing a coefficient of 2 before HCl makes the atoms equal on both sides. It is now a true equation. The following answers are also true equations, but the coefficients are not the smallest possible whole numbers. Therefore, they are not correct answers.

![]() The following unbalanced equation represents a decomposition reaction sometimes used in the laboratory to obtain oxygen gas.
The following unbalanced equation represents a decomposition reaction sometimes used in the laboratory to obtain oxygen gas.
![]()
1. The reactant side has _____ atom(s) of Hg and _____ atom(s) of O.
2. The product side has _____ atom(s) of Hg and _____ atom(s) of O.
Answer: (a) one, one; (b) one, two
![]() The first step in balancing the equation in frame 12 is to make the oxygen atoms on the reactant side equal to the oxygen atoms on the product side. This can be accomplished by placing a coefficient of 2 in front of HgO.
The first step in balancing the equation in frame 12 is to make the oxygen atoms on the reactant side equal to the oxygen atoms on the product side. This can be accomplished by placing a coefficient of 2 in front of HgO.
![]()
The resulting numbers of atoms are:
Reactants |
Products |
2 atoms of Hg |
1 atom of Hg |
2 atoms of O |
2 atoms of O |
Place a coefficient in front of another substance in order to complete the balancing of this equation. _____
Answer: 2HgO → 2Hg + O2 (The equation now indicates two atoms of Hg on the product side as well as on the reactant side.)
![]() The chemical equation 2HgO → 2Hg + O2 is a true equation and is balanced because _______________.
The chemical equation 2HgO → 2Hg + O2 is a true equation and is balanced because _______________.
Answer: the number of each kind of atom is equal on both reactant and product sides of the equation.
![]() The following unbalanced equation represents a single displacement reaction. Add up the atoms on each side on the equation.
The following unbalanced equation represents a single displacement reaction. Add up the atoms on each side on the equation.
![]()
Reactants |
Products |
_______________ atom(s) of Mg |
_______________ atom(s) of Mg |
_______________ atom(s) of H |
_______________ atom(s) of H |
_______________ atom(s) of Cl |
_______________ atom(s) of Cl |
Answer:
Reactants |
Products |
1 atom of Mg |
1 atom of Mg |
1 atom of H |
2 atoms of H |
1 atom of Cl |
2 atoms of Cl |
![]() Use a coefficient to make the chlorine atoms on the reactant side equal to the chlorine atoms on the product side. ______________
Use a coefficient to make the chlorine atoms on the reactant side equal to the chlorine atoms on the product side. ______________
Answer: Mg + 2HCl → MgCl2 + H2 (Place the coefficient of 2 before the HCI.)
![]() Is the equation in frame 16 balanced? (Add the atoms on each side to determine if it is balanced.) ______________
Is the equation in frame 16 balanced? (Add the atoms on each side to determine if it is balanced.) ______________
Answer: yes (Doubling the HCI resulted in doubling both H and Cl atoms on the reactant side; therefore, the equation is balanced.)
![]() The following unbalanced equation represents a combination reaction. Add up the atoms on each side of the equation.
The following unbalanced equation represents a combination reaction. Add up the atoms on each side of the equation.
![]()
Reactants |
Products |
___________________atom(s) of Al |
___________________atom(s) of Al |
___________________atom(s) of O |
___________________atom(s) of O |
Answer:
Reactants |
Products |
1 atom of Al |
2 atoms of Al |
2 atoms of O |
3 atoms of O |
![]() At this point, we can use a coefficient to equalize either the Al atoms or the O atoms. A good rule of thumb to follow is to leave any oxygen or hydrogen atoms until last, placing coefficients elsewhere first. Use a coefficient to equalize the number of atoms of Al on both the reactant and the product side.
At this point, we can use a coefficient to equalize either the Al atoms or the O atoms. A good rule of thumb to follow is to leave any oxygen or hydrogen atoms until last, placing coefficients elsewhere first. Use a coefficient to equalize the number of atoms of Al on both the reactant and the product side.
![]()
Answer: 2Al + O2 → Al2O3 (The numbers of Al atoms are now equal on both sides of the equation, but the equation is not yet balanced.)
![]() Our equation from frame 19 now represents the following number of atoms.
Our equation from frame 19 now represents the following number of atoms.
![]()
Reactants |
Products |
2 atoms of Al |
2 atoms of Al |
2 atoms of O |
3 atoms of O |
1. By what number must the oxygen atoms on the reactant side of the equation be multiplied to equal the oxygen atoms on the product side? __________
2. Is that number a small whole number? ______________
Answer: (a) 1½ (which would result in an equation of 2Al + 1½O2 → Al2O3); (b) no (1½ is not a small whole number.)
![]() The equation in the answer to frame 20 is now balanced. However, the coefficients in chemical equations should normally be whole numbers. We should multiply by the smallest possible whole number to eliminate the fraction. Here, we can multiply every item in the equation by what single whole number? _______________________
The equation in the answer to frame 20 is now balanced. However, the coefficients in chemical equations should normally be whole numbers. We should multiply by the smallest possible whole number to eliminate the fraction. Here, we can multiply every item in the equation by what single whole number? _______________________
Answer: 2 (Multiplying every item by a factor of 2 gives a balanced equation with no fraction.)
![]() Rewrite the equation, multiplying every item by 2. ___________
Rewrite the equation, multiplying every item by 2. ___________
Answer: 4Al + 3O2 → 2Al2O3
![]() The following unbalanced equation represents a single displacement reaction. Determine the number of each kind of atom in the unbalanced equation.
The following unbalanced equation represents a single displacement reaction. Determine the number of each kind of atom in the unbalanced equation.
![]()
Reactants |
Products |
__________atom(s) of Al |
__________atom(s) of Al |
__________atom(s) of H |
__________atom(s) of H |
__________atom(s) of Cl |
__________atom(s) of Cl |
Answer:
Reactants |
Products |
1 atom of Al |
1 atoms of Al |
1 atom of H |
2 atoms of H |
1 atom of Cl |
3 atoms of Cl |
![]() Remember, in balancing equations, it often is useful to leave the H atoms and the O atoms (if any) until last. Rewrite the equation below to equalize the Cl atoms on both sides. ______________________
Remember, in balancing equations, it often is useful to leave the H atoms and the O atoms (if any) until last. Rewrite the equation below to equalize the Cl atoms on both sides. ______________________
![]()
Answer: Al + 3HCl → AlCl3 + H2
![]() Now that the Cl atoms are equal, is the above equation balanced? __________
Now that the Cl atoms are equal, is the above equation balanced? __________
Answer: no (The reactant side has three atoms of H and the product has two atoms of H.)
![]() Look at the following equation.
Look at the following equation.
![]()
What compound or element should be multiplied to make equal numbers of H atoms on both sides of the equation? ______________ By what number? __________
Answer: ![]()
![]() It is usually not acceptable to write a fraction in a chemical equation.
It is usually not acceptable to write a fraction in a chemical equation.
![]()
The fraction can be eliminated with simple math. Multiply every item in the equation by a factor of 2.
Answer: 2Al + 6HCl → 2AlCl3 + 3H2
![]() The above equation is balanced because ______________________.
The above equation is balanced because ______________________.
Answer: it has equal numbers of Al, H, and Cl atoms on each side
![]() The following unbalanced equation represents a combination reaction.
The following unbalanced equation represents a combination reaction.
![]()
1. Determine the number of atoms of each element for each side
Reactants |
Products |
____ atom(s) of P |
____ atom(s) of P |
____ atom(s) of O |
____ atom(s) of O |
____ atom(s) of H |
____ atom(s) of H |
2. Use a coefficient to make the number of P atoms equal on both sides.
Answer:
1. a.
Reactants |
Products |
4 atoms of P |
1 atom of P |
7 atoms of O |
3 atoms of O |
2 atoms of H |
3 atoms of H |
1. b. P4O6 + H2O → 4H3PO3 (representing four molecules of H3PO3)
![]() Now that the P atoms are equal, count the number of atoms on each side of the equation.
Now that the P atoms are equal, count the number of atoms on each side of the equation.
![]()
Reactants |
Products |
____ atom(s) of P |
____ atom(s) of P |
____ atom(s) of O |
____ atom(s) of O |
____ atom(s) of H |
____ atom(s) of H |
Answer:
Reactants |
Products |
4 atoms of P |
4 atoms of P |
7 atoms of O |
12 atoms of O |
2 atoms of H |
12 atoms of H |
![]() Completely balance the equation. (Hint: Equalize the H atoms or the O atoms next.)
Completely balance the equation. (Hint: Equalize the H atoms or the O atoms next.)
![]()
Answer: P4O6 + 6H2O → 4H3PO3
![]() Balance the following equation representing a combination reaction.
Balance the following equation representing a combination reaction.
![]()
Answer:
The first step is to equalize the Cl atoms on both sides.

Then eliminate the fraction.
![]()
![]() Balance the following equation representing a combination reaction. Leave the H and the O atoms until last.
Balance the following equation representing a combination reaction. Leave the H and the O atoms until last.
![]()
Answer: P4O10 + 6H2O → 4H3PO4
![]() The following unbalanced equation represents a double displacement reaction.
The following unbalanced equation represents a double displacement reaction.
![]()
Balance this equation. ________________________________
Answer: BaCl2 + Na2SO4 → BaSO4 + 2NaCl (The only change necessary to balance the equation is placing a coefficient of 2 before NaCl.)
![]() The unbalanced equation below also represents a double displacement reaction.
The unbalanced equation below also represents a double displacement reaction.
![]()
Balance the equation. ____________________
Answer: FeCl3 + 3KOH → Fe(OH)3 + 3KCl
WORD EQUATIONS
![]() Each chemical equation represents a chemical reaction. You have already learned how to name chemical compounds in Chapter 5. Let's apply what you have already learned about naming compounds to reading chemical equations.
Each chemical equation represents a chemical reaction. You have already learned how to name chemical compounds in Chapter 5. Let's apply what you have already learned about naming compounds to reading chemical equations.
The reaction equation below can read as “sodium hydroxide plus hydrogen chloride (or hydrochloric acid) yields sodium chloride and hydrogen oxide (or water).”
NaOH + HCl → NaCl + H2O
The reaction equation below reads as: _____________ plus ____________ yields _____________ plus _____________.
![]()
Answer: silver nitrate; potassium chloride; silver chloride; potassium nitrate
![]() Here are several equations of various types.
Here are several equations of various types.
1. 2P + 5O2 → 2P2O5
2. C + O2 → CO2
3. FeCl3 + 3KOH → Fe(OH)3 + 3KCl
1. Equation 1 reads as __________ plus __________ yields ____________.
2. Equation 2 reads as __________ plus __________ yields ____________.
3. Equation 3 reads as __________ plus __________ yields ____________.
Answer:
1. phosphorus; oxygen; phosphorus(V) oxide (or diphosphorus pentoxide)
2. carbon; oxygen; carbon dioxide (or carbon(IV) oxide)
3. iron(III) chloride (or ferric chloride); potassium hydroxide; iron(III) hydroxide (or ferric hydroxide); potassium chloride
![]() Write the balanced equation for sodium plus water yields sodium hydroxide plus hydrogen gas. (Hydrogen gas is H2.) ________________________
Write the balanced equation for sodium plus water yields sodium hydroxide plus hydrogen gas. (Hydrogen gas is H2.) ________________________
Answer:
The unbalanced equation is Na + H2O → NaOH + H2
The balanced equation is 2Na + 2H2O → 2NaOH + H2
![]() Write the balanced equation for potassium hydroxide plus hydrochloric acid yields potassium chloride plus water. ______________________
Write the balanced equation for potassium hydroxide plus hydrochloric acid yields potassium chloride plus water. ______________________
Answer: KOH + HCl → KCl + H2O
You have had some practice in balancing equations that represent several types of reactions. In the next section you will learn how to predict whether or not a reaction will actually occur as written in the equation.
REACTIONS: GO OR NO GO
![]() Many of the reaction equations you have balanced thus far involve ionic compounds and take place in water (aqueous) solutions. The ionic compounds in aqueous solution actually dissociate, meaning that the negative and positive ions separate and move about freely in the solution.
Many of the reaction equations you have balanced thus far involve ionic compounds and take place in water (aqueous) solutions. The ionic compounds in aqueous solution actually dissociate, meaning that the negative and positive ions separate and move about freely in the solution.
The reaction equation from frame 39 can also be written in complete ionic form.
![]()
Which item in the complete ionic equation is obviously not an ion but is a covalently bonded molecule? ___________________
Answer: H2O
![]() In the ionic equation in frame 40, the whole reaction takes place in water (aqueous) solution. All of the ions are completely dissolved in water. We can show that the reaction takes place in water by writing (aq), which means “aqueous,” behind every ion in the equation.
In the ionic equation in frame 40, the whole reaction takes place in water (aqueous) solution. All of the ions are completely dissolved in water. We can show that the reaction takes place in water by writing (aq), which means “aqueous,” behind every ion in the equation.
![]()
Not only did the reaction take place in water (aqueous) solution, but water is also a product. Note that (l) is placed after H2O to indicate that it is a liquid. The formation of water as a product is one way to know whether a reaction actually occurs.
A second way to know that a reaction has occurred is if a solid precipitate is made. A precipitate is a compound that does not dissolve in water. If a precipitate is formed as a product in a reaction that is taking place in water solution, the precipitate will come out of the solution and will normally settle to the bottom of the solution container. In an experimental situation, it is easy to see if a precipitate is formed. We can also use a solubility table, such as the table (see page 143) to predict whether or not a precipitate will form.
What are two ways to tell whether or not a reaction will take place? ____________________
Answer: A reaction will take place if either of the following is true: (1) if water (H2O) is a product or (2) if a precipitate is a product.
![]() If H2O or a precipitate is not formed as a product, or if other products that will be covered later are not formed, we may just simply have an aqueous solution of the various ions with no reaction taking place at all. It is, therefore, important to determine if a reaction is likely to produce a precipitate. The table above is very useful for determining whether or not a compound formed from a pair of ions is soluble or insoluble in water. Insoluble compounds are precipitates. To use the table, first determine which ions make up the compound. As you learned in Chapter 3, an ionic compound is made up of a positive ion, which is usually a metal or H+ or
If H2O or a precipitate is not formed as a product, or if other products that will be covered later are not formed, we may just simply have an aqueous solution of the various ions with no reaction taking place at all. It is, therefore, important to determine if a reaction is likely to produce a precipitate. The table above is very useful for determining whether or not a compound formed from a pair of ions is soluble or insoluble in water. Insoluble compounds are precipitates. To use the table, first determine which ions make up the compound. As you learned in Chapter 3, an ionic compound is made up of a positive ion, which is usually a metal or H+ or ![]() , and a negative ion, which is usually the remainder of the compound and is made up of one or more nonmetal atoms.
, and a negative ion, which is usually the remainder of the compound and is made up of one or more nonmetal atoms.
After determining the ion pair that makes up the compound, locate the ions on the table and determine if the ion pair will form a precipitate or if the compound is soluble. For example, the compound CaCl2 is made up of Ca2+ and Cl− ions. According to the fourth statement on the table, all compounds made up of Cl− ions are soluble except when combined with Ag+, Hg2+, or Pb2+. Since Cl− is combined with Ca2+, the compound is soluble.
Using the table, underline those compounds that are insoluble and would therefore precipitate in aqueous solution.
![]()
Answer: AgCI, CaCO3, and Mg(OH)2
TABLE OF SOLUBILITY OF SOME COMMON COMPOUNDS
· All common compounds made up of alkali metal ions (Li+, Na+, K+, Rb+, Cs+, or Fr+) or ![]() and negative ions are soluble.
and negative ions are soluble.
· All compounds containing ![]() or
or ![]() and a positive ion are soluble.
and a positive ion are soluble.
· All compounds containing ![]() are soluble except when combined with ions of Ba2+, Sr2+, or Pb2+.
are soluble except when combined with ions of Ba2+, Sr2+, or Pb2+.
· All compounds containing Br−, Cl−, or I− are soluble except when combined with ions of Ag+, ![]() , or Pb2+.
, or Pb2+.
· Compounds of ![]() with alkaline earth metal ions (Be2+, Mg2+, Ca2+, Sr2+, Ba2+, or Ra2+) and alkali metal ions (Li+, Na+, K+, Rb+, Cs+, or Fr+) or
with alkaline earth metal ions (Be2+, Mg2+, Ca2+, Sr2+, Ba2+, or Ra2+) and alkali metal ions (Li+, Na+, K+, Rb+, Cs+, or Fr+) or ![]() are soluble. Compounds of
are soluble. Compounds of ![]() are insoluble with all other positive ions.
are insoluble with all other positive ions.
· Compounds with OH— are soluble only with Ba2+, Li+, Na+, K+, Rb+, Cs+, Fr+, or ![]() . Compounds with OH− are insoluble with all other positive ions.
. Compounds with OH− are insoluble with all other positive ions.
· All compounds with ![]() ,
, ![]() , or S2− are insoluble except with alkali metal ions (Li+, Na+, K+, Rb+, Cs+, or Fr+) or
, or S2− are insoluble except with alkali metal ions (Li+, Na+, K+, Rb+, Cs+, or Fr+) or ![]() .
.
![]() Underline those compounds listed below that are insoluble and will form precipitates in aqueous solution.
Underline those compounds listed below that are insoluble and will form precipitates in aqueous solution.
![]()
Answer: PbSO4, Hg2I2, and MgCO3
![]() Does the following reaction actually occur? Why or why not? (Hint: Use the table to check to see if one of the products is a precipitate.) __________________________________________________
Does the following reaction actually occur? Why or why not? (Hint: Use the table to check to see if one of the products is a precipitate.) __________________________________________________
![]()
Answer: Yes, the reaction occurs because AgI will precipitate.
![]() Does the following reaction occur? Why or why not? _______________________________________________
Does the following reaction occur? Why or why not? _______________________________________________
![]()
Answer: Yes, the reaction occurs because both products are precipitates.
![]() You have already learned two means for determining whether or not a reaction occurs. If either water or a precipitate is a product, a reaction occurs. Other means for determining if a reaction actually occurs are the formation of a gas, a weak electrolyte, or a covalent compound. In an experiment, it is easy to detect a gas as a product since it usually bubbles up from an aqueous solution. Typical gases that you have encountered several times in this and previous chapters are H2 (hydrogen gas), O2 (oxygen gas), and CO2 (carbon dioxide gas). We will specify if gases other than these are products.
You have already learned two means for determining whether or not a reaction occurs. If either water or a precipitate is a product, a reaction occurs. Other means for determining if a reaction actually occurs are the formation of a gas, a weak electrolyte, or a covalent compound. In an experiment, it is easy to detect a gas as a product since it usually bubbles up from an aqueous solution. Typical gases that you have encountered several times in this and previous chapters are H2 (hydrogen gas), O2 (oxygen gas), and CO2 (carbon dioxide gas). We will specify if gases other than these are products.
Weak electrolytes are ionic compounds that dissociate only partially and, therefore, only conduct a weak electric current in aqueous solutions. We will specify if any products are weak electrolytes. (Later chapters will deal more specifically with weak electrolytes.)
Covalent compounds were described in Chapter 3 as compounds that are not ionic and typically are composed of nonmetal atoms. Typical covalent compounds include both H2O and the gases listed above. We will specify if covalent compounds other than these are reaction products.
If none of these products is formed, assume that a reaction does not occur at all. Does the following reaction occur? Why or why not?
![]()
Answer: Yes, the reaction occurs because a gas (H2) is formed.
![]() In the following equation, the products listed are both strong electrolytes (therefore not weak electrolytes). There are no gases or covalent compounds produced. Does the reaction occur? Why or why not?
In the following equation, the products listed are both strong electrolytes (therefore not weak electrolytes). There are no gases or covalent compounds produced. Does the reaction occur? Why or why not?
![]()
Answer: No, the reaction does not occur. The question has already eliminated gases and covalent compounds. Since the products are strong electrolytes, they cannot be weak electrolytes. Water is not a product. Neither of the possible products is a precipitate (according to the solubility table). Therefore, the reaction does not occur.
![]() Here is another double displacement chemical equation. No weak electrolytes, gases, or covalent compounds are produced. Does the reaction occur? Why or why not?
Here is another double displacement chemical equation. No weak electrolytes, gases, or covalent compounds are produced. Does the reaction occur? Why or why not?
![]()
Answer: Yes, the reaction occurs. Although the products are not weak electrolytes, gases, water, or other covalent compounds, one of the products (PbBr2) is a precipitate.
IONIC EQUATIONS
![]() Although most of the previous equations involving ions in aqueous solution have been written in molecular form (showing complete chemical formulas), a more correct method would be to write an equation showing the ions as dissociated. The following equation is in molecular form.
Although most of the previous equations involving ions in aqueous solution have been written in molecular form (showing complete chemical formulas), a more correct method would be to write an equation showing the ions as dissociated. The following equation is in molecular form.
![]()
A more correct method of writing the same equation would show the reactants as ions. In the following equation (reactant side only), note that water is neither a reactant nor a product. Although the reaction takes place in water solution, it does not participate as either a reactant or a product; therefore it can either be left out of the equation or the abbreviation (aq) can be placed beside each ion. Complete the reactant side of this equation in ionic form.
![]()
Answer: ![]()
![]() The complete molecular equation is AgNO3 + NaCl → AgCl + NaNO3. Since the AgCl is a precipitate and remains an undissolved solid, we place (s) beside its formula (which stands for solid). Write the complete ionic equation representing the precipitate and all ions in aqueous solution.
The complete molecular equation is AgNO3 + NaCl → AgCl + NaNO3. Since the AgCl is a precipitate and remains an undissolved solid, we place (s) beside its formula (which stands for solid). Write the complete ionic equation representing the precipitate and all ions in aqueous solution.
![]()
Answer: ![]()
![]() The equation in frame 50 is called a complete ionic equation. A complete ionic equation usually includes some ions that did not take part in the reaction and can be found on both sides of the equation. Those ions that do not take part in the reaction are called spectator ions.
The equation in frame 50 is called a complete ionic equation. A complete ionic equation usually includes some ions that did not take part in the reaction and can be found on both sides of the equation. Those ions that do not take part in the reaction are called spectator ions.
Look at the complete ionic equation again.
![]()
The spectator ions are ____ and ____.
Answer: ![]()
![]() A complete ionic equation contains all ions including spectator ions. If the spectator ions are eliminated from both sides of a complete ionic equation, the result is called a net ionic equation.
A complete ionic equation contains all ions including spectator ions. If the spectator ions are eliminated from both sides of a complete ionic equation, the result is called a net ionic equation.
For the same complete ionic equation, write the net ionic equation.
![]()
Answer: Ag+(aq) + Cl−(aq) → AgCl(s)
![]() A net ionic equation contains no spectator ions. A complete ionic equation includes spectator ions. When NaOH is added to FeCl3 in aqueous solution, a precipitate Fe(OH)3 forms. What ions would be included in the complete ionic equation for this reaction? (Forget about balancing the equation for the moment.)
A net ionic equation contains no spectator ions. A complete ionic equation includes spectator ions. When NaOH is added to FeCl3 in aqueous solution, a precipitate Fe(OH)3 forms. What ions would be included in the complete ionic equation for this reaction? (Forget about balancing the equation for the moment.)
![]()
Answer: Na+(aq) + OH−(aq) + Fe3+(aq) + Cl−(aq) → Fe(OH)3(s) + Na+(aq) + Cl−(aq)
![]() Now balance the equation in frame 53 so that there are equal numbers of elements (including ions) on each side. (Hint: You may find it easier to balance the molecular version first. The unbalanced molecular version is NaOH + FeCl3 → Fe(OH)3 + NaCl.)
Now balance the equation in frame 53 so that there are equal numbers of elements (including ions) on each side. (Hint: You may find it easier to balance the molecular version first. The unbalanced molecular version is NaOH + FeCl3 → Fe(OH)3 + NaCl.)

Answer: The balanced molecular version is
![]()
The balanced complete ionic equation is
![]()
![]() Eliminate the spectator ions on both sides of the ionic equation in frame 54. The resulting equation is:
Eliminate the spectator ions on both sides of the ionic equation in frame 54. The resulting equation is:
The resulting equation is called a(n) ________________ ionic equation.
Answer: Fe3+(aq) + 3OH−(aq) → Fe(OH)3(s); net ionic
![]() An aqueous solution of Na2CO3 is added to dilute hydrochloric acid. The resulting carbonic acid, H2CO3, breaks down to form CO2 gas and H2O. Complete and balance the molecular equation for this reaction.
An aqueous solution of Na2CO3 is added to dilute hydrochloric acid. The resulting carbonic acid, H2CO3, breaks down to form CO2 gas and H2O. Complete and balance the molecular equation for this reaction.
![]()
Answer: Na2CO3 + 2HCl → NaCl + CO2 + H2O
![]() Write the complete ionic equation representing the reaction in frame 56. CO2 escapes as a gas; therefore we place (g) behind its formula. Both CO2 and H2O are considered to be covalent molecules; therefore CO2 and H2O remain in molecular form.
Write the complete ionic equation representing the reaction in frame 56. CO2 escapes as a gas; therefore we place (g) behind its formula. Both CO2 and H2O are considered to be covalent molecules; therefore CO2 and H2O remain in molecular form.
Answer: ![]()
![]() Write the net ionic version of the equation in frame 57.
Write the net ionic version of the equation in frame 57.
Answer: ![]()
![]() Complete the following double displacement reaction in molecular form and balance.
Complete the following double displacement reaction in molecular form and balance.
![]()
Answer: CuSO4 + 2LiOH → Cu(OH)2 + Li2SO4
![]() What ions are represented on the left (reactant) side of the equation in frame 59?
What ions are represented on the left (reactant) side of the equation in frame 59?
____________ + _____________ + _____________ + ____________ →
Answer: ![]()
![]() Do any pair of the four ions in frame 60 form an insoluble precipitate? (Use the table on page 143.) ____________ If yes, what pair of ions? ______________
Do any pair of the four ions in frame 60 form an insoluble precipitate? (Use the table on page 143.) ____________ If yes, what pair of ions? ______________
Answer: yes; Cu2+ and 2OH− form an insoluble precipitate: Cu(OH)2.
![]() Finish the complete ionic equation below. The precipitate should be in molecular form.
Finish the complete ionic equation below. The precipitate should be in molecular form.
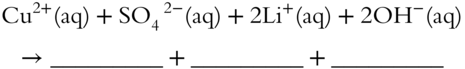
Answer: ![]()
![]() Write the net ionic equation for the equation in frame 62.
Write the net ionic equation for the equation in frame 62.
Answer: Cu2+(aq) + 2OH−(aq) → Cu(OH)2(s)
![]() Complete the following for the reaction of silver sulfate and sodium iodide.
Complete the following for the reaction of silver sulfate and sodium iodide.
1. Complete and balance the following molecular equation.
Ag2SO4 + NaI → ___________ + ___________
2. What ions are represented on the reactant side of the above equation? ____________ + ____________ + ____________ + ____________ →
3. No gases, weak electrolytes, or covalent compounds are products. Will the reaction occur? Why or why not? _______________________
4. Write the complete ionic equation. _____________________________________________________
5. Write the net ionic equation. _____________________________________________________
Answers:
1. Ag2SO4 + 2NaI → 2AgI + Na2SO4
2. ![]()
3. Yes, Ag+ and I− form a precipitate (AgI).
4. ![]()
5. 2Ag+(aq) + 2I−(aq) → 2AgI(s)
We can simplify this net ionic equation by dividing by a factor of 2. Thus: Ag+(aq) + I−(aq) → AgI(s). Either answer is acceptable.
![]() Let's try this again with another reaction.
Let's try this again with another reaction.
1. Complete and balance the following double displacement reaction in molecular form.
BaCl2 + Na2SO4 → ______________ + ______________
2. Does the reaction occur even though there are no gases, weak electrolytes, or covalent compounds formed? Why or why not? ______________________
3. Write the complete ionic equation for the above. _____________________________________________
4. Write the net ionic equation. _______________________________________________
Answers:
1. BaCl2 + Na2SO4 → BaSO4 + 2NaCl
2. Yes, BaSO4 precipitates.
3. ![]()
4. ![]()
![]() Here is another reaction to figure out.
Here is another reaction to figure out.
1. Complete and balance the following molecular equation. AgNO3 + KCl → ___________________
2. No product is a gas, a weak electrolyte, or a covalent compound. Does this reaction occur? Why or why not? __________________________
3. Write the complete ionic equation for the above. _______________________________________________________
4. Write the net ionic equation. ______________________________________________
Answers:
1. AgNO3 + KCl → AgCl + KNO3
2. Yes, AgCI precipitates.
3. ![]()
4. Ag+(aq) + Cl−(aq) → AgCl(s)
![]() One more time.
One more time.
1. Complete and balance the following molecular equation. One of the products of this reaction is H2CO3, which immediately breaks down to form H2O and CO2.
![]()
2. Write the complete ionic equation for this reaction. ___________________________________________________
3. Write the net ionic equation. _______________________________________________________
Answers:
1. K2CO3 + H2SO4 → K2SO4 + H2CO3
(Since the H2CO3 breaks down into CO2 and H2O immediately, it should be written as follows: K2CO3 + H2SO4 → K2SO4 + H2O + CO2.)
2. ![]()
3. ![]()
Chemical formulas and equations are extremely useful tools for chemists. They save a lot of time and words when we try to communicate. You will encounter chemical equations throughout the rest of this book, so it is important that you know the material in this chapter.
In the next chapter you will use balanced chemical equations to examine weight relationships between reactants and products. (Ions and ionic equations will appear again in Chapters 9 and 11 through 13, so you can see how important they are to your understanding of chemistry.)
SELF-TEST
This self-test is designed to show how well you have mastered this chapter's objectives. Correct answers and review instructions follow the test.
1. What coefficients provide a balanced reaction for the neutralization of sodium hydroxide with sulfuric acid? NaOH + H2SO4 → H2O + Na2SO4
2. What coefficients provide a balanced reaction for the neutralization of magnesium hydroxide with phosphoric acid? Mg(OH)2 + H3PO4 → H2O + Mg3(PO4)2
3. Circle the reaction that is correct for the decomposition of ozone (O3) to oxygen gas.
![]()
![]()
![]()
4. Circle the reaction that is correct for the oxidation of glucose (C6H12O6) to water and carbon dioxide gas.
![]()
![]()
![]()
5. Identify the ions that are always soluble in solution. Li+, ![]() ,
, ![]() , S2−
, S2−
6. Identify the ions that are sometimes insoluble in solution, depending on the metal ion found. ![]() ,
, ![]() , OH−, Cl−, K+,
, OH−, Cl−, K+, ![]() ,
, ![]()
7. Complete and balance the following reactions as molecular equations, complete ionic equations, and net ionic equations.
1. ammonium hydroxide + sulfuric acid→ _____________________________________________________________
2. lead(II) nitrate + sodium chloride→
3. Fe2(SO4)3 + Ba(OH)2→
8. Complete and balance the following reactions as molecular equations, complete ionic equations, and net ionic equations.
1. lithium hydroxide + magnesium nitrate→ _____________________________________________________________
2. lead(II) nitrate + potassium iodide→
9. Are the reactions that produce a precipitate a type of single displacement or double displacement? _________________________
10. Indicate whether each of the following reactions is combination, decomposition, single displacement, or double displacement.
1. 2HBr → H2 + Br2__________________________
2. 4Ag + O2 → 2Ag2O__________________________
3. 2Mg + CO2 → 2MgO + C__________________________
11. Indicate whether each of the following reactions is combination, decomposition, single displacement, or double displacement.
1. NaCl + KBr → NaBr + KCl__________________________
2. Zn + 2HCl → ZnCl2 + H2__________________________
3. CO + Cl2 → COCl2__________________________
12. Using the table on page 143, underline the soluble compounds in the list below.
![]()
13. Using the table on page 143, underline the insoluble compounds in the list below.
![]()
14. Do all the equations in question 7 occur? Why or why not?
1. __________________________
2. __________________________
3. __________________________
15. A general chemistry student mixed several different ionic solutions together in a large beaker. The mixture contained S2−, OH−, ![]() , and
, and ![]() . However, she noticed that nothing had happened upon mixing (i.e., no solids formed). What can the student infer about the ions from these results?
. However, she noticed that nothing had happened upon mixing (i.e., no solids formed). What can the student infer about the ions from these results?
ANSWERS
Compare your answers to the self-test with those given below. If you answer all questions correctly, you are ready to proceed to the next chapter. If you miss any, review the frames indicated in parentheses following the answers. If you miss several questions, you should probably reread the chapter carefully.
1. 2NaOH + H2SO4 → 2H2O + Na2SO4 (frames 8—35)
2. 3Mg(OH)2 + 2H3PO4 → 6H2O + Mg3(PO4)2 (frames 8—35)
3. 2O3 → 3O2 (frame 11)
4. C6H12O6 + 6O2 → 6H2O + 6CO2 (frame 11)
5. Li+ and ![]() (frame 41)
(frame 41)
6. OH−, Cl−, ![]() (frame 41)
(frame 41)
7.
1. 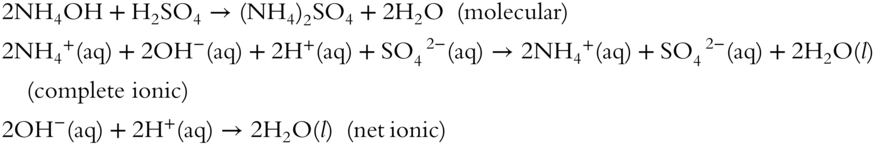
2. 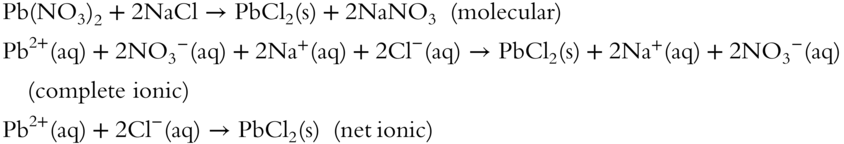
3. 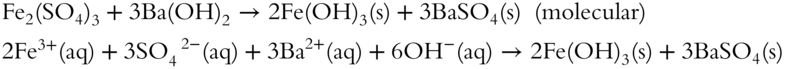 (complete and net ionic because both products are precipitates)
(complete and net ionic because both products are precipitates)
(frames 6—39, 49—67)
8.
1. 
2.  (frames 6—39, 49—67)
(frames 6—39, 49—67)
9. Double displacement (frames 1—5)
10. (a) decomposition; (b) combination; (c) single displacement (frames 1—5)
11. (a) double displacement; (b) single displacement; (c) combination (frames 1—5)
12. NaHCO3, KOH, NH4Br, AgNO3 (frames 41—43)
13. AgCI, CuS (frames 41—43)
14.
1. yes, because H2O is produced
2. yes, because the precipitate PbCl2 is formed
3. yes, because both products are precipitates (frames 40—48)
15. Since no solids formed the student must have had some mixture of alkali metals and/or ammonium ions in the solution (frames 6—39, 49—67, and solubility table on page 143)