Mathematics of Life (2011)
Chapter 2. Creatures Small and Smaller
If human eyesight had been better, we might never have experienced the first revolution, when we noticed the hidden wonders of life. Our poor eyesight inspired a simple piece of technology – the lens. Unexpectedly, this practical aid to our everyday activities spun off two types of scientific instrument: the telescope and the microscope. These opened up the vast reaches of the cosmos and the intricate small-scale world of living creatures.
Unaided, the human eye sees the world on a human scale: people, houses, animals, plants, rocks, rivers, cups, knives ... Even the larger features of our environment – mountains, lakes – we perceive as monolithic objects. From a distance, a mountain looks much like a rock, one that comes to a point at the top. By the time we are close enough to see how much more there is to a mountain, we have lost sight of the mountain. Instead, we see a complex arrangement of streams, scattered rocks, moss, precipices, ravines, snow and ice.
The word ‘grasp’ gives away the whole game. On a human scale, the world consists of what we can pick up with our hands. On this level, the Moon, a cow and a flea seem to be on a par with one another. Agreed, we can’t grasp the Moon, but we can cover it with a thumb held at arm’s length. We can’t pick up a cow, but we can put a ring through its nose and lead it on a rope. (I use ‘we’ in the time-honoured sense of ‘some of us can’.) The main problem in grasping a flea, ironically, is that it’s too small to offer a good grip – and it jumps. But broadly speaking, on a human scale all objects are on much the same footing. We give them a name, and we imagine that by naming them we have captured their essence. The Moon is a shining, mottled disc. A cow walks on four legs and gives milk. A flea bites, jumps and is a nuisance.
As soon as we progress beyond the unaided human eye, with little more than a polished lump of glass to assist us, our simple, comfortable picture of the world changes. Through his telescope Galileo saw spots on the Sun, mountains on the Moon, phases of Venus, and four tiny specks of light passing to and fro across the orange disc of the planet Jupiter. He deduced – could scarcely fail to deduce, as soon as he put his mind to it – that the Sun and Moon are not unblemished spheres, Venus revolves around the Sun, and the Earth is not a fixed centre around which the rest of the universe revolves.
The religious authorities of the time, who considered themselves to be custodians of the truth, were aghast. Galileo managed to escape the horrific penalties that were often employed to enforce the official view of truth, but at his trial for heresy in 1633 he was forced to deny his own deductions from the evidence that he had seen through his telescope. The authorities of the day did not dispute the evidence. They simply told Galileo to ignore it, and stop writing about it. I’m inclined to believe that they acted like this not because they were religious, but because they were authorities.
So Galileo recanted, though allegedly muttering under his breath ‘even so, it moves’. And the Earth continued to move round the Sun, whatever the Church believed and whatever Galileo was publicly obliged to assert. The scientific evidence eventually prevailed, but by the time Pope John Paul II apologised for how Galileo had been treated, science had put men on the Moon.
If a humble telescope could cause such ructions, merely by revealing things that were there, what about the microscope? That opened up the internal world of very small things – in particular, living creatures. The potential for heretical ideas was far greater than anything that astronomy could inspire. Yet curiously, the religious authorities viewed this even more revolutionary development with apparent equanimity, even though the new evidence now made available to the human eye would totally change our ideas about the world and our place in it. I suspect that the authorities simply didn’t grasp the microscope’s potential. The wonders it revealed did not, initially, appear to conflict with scripture. The Church, taking a positive religious message, believed the microscope was merely showing us the hidden marvels of God’s creation. A pity they didn’t think the same about Galileo.
In fact, the microscope was far from innocuous. It quickly revealed that our world is not what it seems. It does not function solely on the human level, it was not made for humans; everything that humans had been taking for granted about plants and animals was up for grabs, and most of it was wrong. Even those things, like cats and cows and trees, that do seem to function on a human level ... don’t.
On the human level, a cow seems simple. You feed it grass, and it pays you back with milk. It’s a trick whose secret is limited to cows and a few other mammals (most can’t digest grass). You don’t need to understand the details to exploit the process: it’s a straightforward transformation from grass into milk, more like chemistry – or alchemy – than biology. It is, in its way, magic, but it’s rational magic that works reliably. All you need is some grass, a cow and several generations of practical knowhow.
Seen through a microscope, though, it all gets more complicated. And the closer you look, the more complicated it gets. Milk is not a single substance, but a mixture of many. Grass is so complex that we still don’t fully understand it. A cow’s complexity is even greater. In particular, a cow (plus a bull) can make a new generation of baby cows. This is a simple thing on a human level, but inexpressibly complex on a microscopic level.
Nearly three thousand years ago, the ancient Egyptians knew that a glass lens can make an object look bigger. Seneca, who tutored the Roman Emperor Nero, noticed that it is easier to read someone’s writing if you look through a glass globe filled with water. Nero himself is said to have looked through an emerald to watch his gladiators fighting in the arena. By the ninth century, people were using ‘reading stones’ to assist their failing eyesight. These were polished lumps of clear glass, rounded on one side and flat on the other; you sat them on top of the document you were trying to read and looked through them. By the twelfth century, the Chinese had discovered that slices of smoky quartz can protect your eyes from the sun.
No one knows exactly when, where or by whom the first true spectacles – a pair of lenses that you perch on your nose – were invented. One contender is Salvino D’Armati, who lived in Florence and may have invented spectacles around 1284. Another is a Dominican monk, Alessandro Spina, from Pisa. A third is Roger Bacon, whose 1235 (or earlier) book about the rainbow mentions using optical devices to read small letters from a distance. However, we have no idea what sort of device he had in mind. It may have been just a single crude lens.
Whoever should be given the credit, the first true spectacles were almost certainly invented in Italy between 1280 and 1300. They acted like a magnifying glass and corrected long-sightedness; it would be another 300 years before lenses able to correct shortsightedness were developed, in part because these are much harder to make. Johannes Kepler (astronomer, astrologer and mathematician) was the first to explain how convex and concave lenses corrected eyesight. Spectacles work better if the lenses are made from clear glass, without too many bubbles or impurities, and the precise shape of the lens is crucial. Lenses were (and still are) made by grinding glass using various types of abrasive material, which in Kepler’s time were already being used by jewellers. So lens technology developed alongside other improvements.
In 1590 a Dutch spectacle manufacturer, Zaccharias Janssen, assisted by his son Hans, put several lenses inside a tube. When they looked through the tube, it made everything appear larger and nearer. This discovery led to two of the most important scientific instruments ever invented: the telescope and the microscope. The telescope brought the large, distant structures of the cosmos down to a human scale. The microscope did the exact opposite: it took the diminutive structures of Earthly objects, especially living creatures, and brought them up to the human scale.
By 1609 Galileo had improved these early telescopes, and through his still rather crude instruments he made discoveries that persuaded him that the Earth was not the centre of the universe. Within a century, astronomy had become a thriving area of science, and the secrets of the heavens, especially the laws of gravity, were there for the taking.
The telescope opened up astronomy because it made it possible for the human eye to see enormously distant, enormously large objects, such as planets. It took the exact opposite to open up biology: a device that allowed the human eye to see incredibly tiny objects that were right in front of people’s noses. By a happy accident, the same basic technology – lenses – did this job too. The resulting device even has a similar name: the microscope.
The invention of the microscope had a very different effect from that of the telescope. It led to great strides in biology, but instead of clarifying the issues, many of those strides made things seem even more mysterious and miraculous. Instead of opening up the world of living creatures to human understanding, the microscope just made the puzzles seem even more difficult. Through even a low-powered microscope, little more than a single crude lens, living creatures took on new significance. And they were very, very complex.
So, while the telescope revealed deep simplicities in the cosmos, the microscope revealed previously unseen complexities in life. The same dichotomy between the simple and the complex has bedevilled the biological sciences ever since. Biologists, with some justification, argue that the life sciences are fundamentally harder than the physical sciences.
A key figure in the development of the microscope was the Dutch tradesman and scientist Anton van Leeuwenhoek. He developed a way to make small, high-quality spheres of glass, and used them as lenses. Although a sphere is not the ideal shape for a lens, the quality of the glass compensated for the poor geometry, and van Leeuwenhoek’s microscopes were surprisingly powerful. Using this new device, he became the first person to observe bacteria, yeast and microscopic creatures that dwelt in ponds. Under one of his microscopes, a drop of pondwater teemed with as much life as the Serengeti plain. He also discovered that blood was made from tiny disc-shaped objects, which flowed round the body in tiny tubes, capillaries.
Starting in 1673, van Leeuwenhoek published his discoveries in the Philosophical Transactions, a journal of the Royal Society in London. At first his work attracted favourable comment, but after three years he began to make claims that most scientists of the day found absurd: the discovery of ‘animalcules’. These creatures, he said, flourished inside a single drop of water. The idea that there might exist living organisms so small that they were invisible to the naked eye seemed ludicrous, and at first van Leeuwenhoek’s claim was met with derision.
The types of creature that van Leeuwenhoek discovered are nowadays known as protists. The best known protist must be ‘the’ amoeba, thanks to school biology; actually there are innumerable species of amoeba, some of which even have shells. So ‘amoeba’ has become a generic term for all such creatures (the technical term is ‘amoeboid’). Amoebas were discovered in 1757 by August von Rosenhof, and initially they were referred to as ‘Proteus animalcules’, after the Greek god famed for his ability to change shape. The amoeba with the scientific name Amoeba proteus is the most familiar, mainly because it is also one of the largest, and so can easily be seen under a low-powered microscope (see Figure 1).
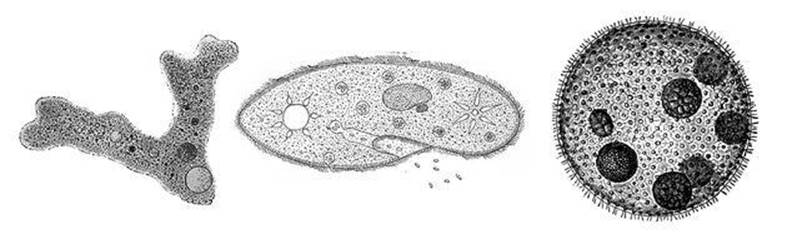
Fig 1 Left to right: amoeba, Paramecium, Volvox.
When so viewed, this particular amoeba appears as an irregularly shaped blob with several protrusions, like rudimentary tentacles, with a rather rounded shape. The outside of the creature is some sort of membrane, forming a flexible bag; the inside is a mixture of various granules, and a few holes, which flow with apparent purpose, like a thick jelly dotted with grains of sand that seem to know where they want to go. One rounded feature, dotted with even smaller particles, stands out: this is the nucleus. An amoeba can move and ingest food, and thanks to its nucleus it can even reproduce – its famous ability to ‘multiply by division’. Under the right conditions the nucleus orchestrates a complex sequence of events that cause one amoeba to split into two. These in turn can grow, and divide again, so the amoeba’s lineage can flourish.
One of my favourite cartoons shows the archetypal Noah’s Ark, propped up by wooden scaffolding, the rain bucketing down. The last few pairs of animals are making their way up the gangplank into the ark, wet and miserable. Noah is grubbing around in the mud at the foot of the gangplank, desperately looking for something. Mrs Noah is leaning over the side of the ark, shouting: ‘Noah! Forget the other amoeba!’
Van Leeuwenhoek also saw Paramecium, a slipper-shaped organism covered in tiny whip-like protrusions known as cilia (plural of cilium). These undergo wave-like motions and move the animal around. Paramecium also has a surrounding membrane. There is a mouth-like groove at one end and an anal pore at the other. It also has a relatively large nucleus, now called a macronucleus because genetically it resembles a large number of distinct nuclei that have merged into a single body.
A third common inhabitant of water-drops is a plant: Volvox. A mature Volvox is a colony of single-celled algae, and each cell propels itself with a flagellum, a tail-like object which appears to wiggle from side to side. These colonies, which can number up to 50,000 individuals, are contained in a larger (though still microscopic) sphere made from a gelatinous protein. They are bright green because they contain chlorophyll, the substance that gives plants their green colour and, more crucially, allows them to turn sunlight into chemical energy.
All this, and much more, inside one drop of water? It was scarcely credible. The luminaries of the Royal Society found it wildly unlikely, but after a further four years people started looking for themselves instead of denouncing the idea as absurd. Van Leeuwenhoek was vindicated, and soon he was elected a Fellow of the Society.
He made a number of fundamental discoveries using his microscopes, but ultimately his most important works were the microscopes themselves, because other people could use them to make their own discoveries. Van Leeuwenhoek manufactured more than 500 lenses and built 400 different microscopes. The best of the nine surviving models magnifies objects up to 275 times, and some of his models may have been capable of 500-fold magnification. This is five times more than a standard modern laboratory optical microscope. Of course, today’s microscopes are manufactured with greater precision, and include all sorts of extras, and much higher magnification is available if you really need it. But you can do a lot of biology with one of Van Leeuwenhoek’s microscopes.
Van Leeuwenhoek was a Calvinist, and considered his discoveries to be evidence of the hidden wonders of God’s creation. On a scientific level he disproved the prevailing belief that microscopic organisms were ‘spontaneously generated’ – arose from non-living materials of their own accord – by showing that, just like larger living creatures, they reproduced. It is ironic that the telescope, with which Galileo revealed new things about the distant cosmos, raised so many religious hackles, but the microscope, which opened up entirely new visions of life on this planet, was accepted without a qualm.
It was not to last, of course. But the deep religious and emotional divisions that would be triggered by Darwin and his successors lay 200 years in the future.
Microscopy really began to take off, and biology with it, when Robert Hooke joined the fray. Hooke was an English polymath and natural philosopher – the term used in those days for ‘scientist’ – and he took up where Van Leeuwenhoek left off. He was in many ways the true father of microscopy. He was into everything, and he possessed immense energy. When Hooke embarked on a new project, the sparks flew.
Hooke was responsible for one of the iconic biological drawings, one that made a very clear point about the complexities of minute organisms. In his lavishly illustrated Micrographia of 1665 he presented observations that he had made with both microscope and telescope. One of the engravings shows what a flea looks like through a modestly powered microscope (see Figure 2). All his contemporaries were familiar with fleas, indeed on intimate terms with them, but to most people these irritating little beasts were just dark specks that jumped a lot and sucked blood. Hooke revealed how complex a flea really is. It looks like a diminutive armoured machine. It has long legs, which allow it to jump, and the legs are hairy. Its mouth parts, which suck the blood, are surprisingly complicated. Clearly there is more to a flea than just being a nuisance.
Hooke was responsible for an even more iconic drawing, also in the Micrographia. It showed a thin slice through an everyday substance, cork (see Figure 3). Cork is the bark of a tree, and it is strong and light. These two properties stem from its microscopic structure: it consists of innumerable tiny chambers. Hooke called these chambers ‘cells’ because they reminded him of the rooms inhabited by monks. Cells are the basic building blocks of life.
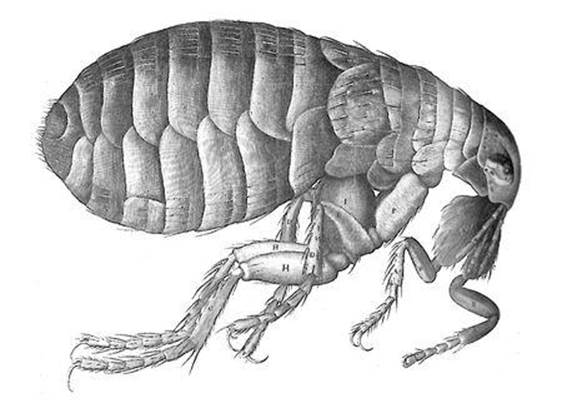
Fig 2 Hooke’s drawing of a flea, from Micrographia.
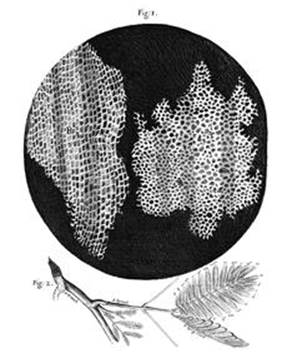
Fig 3 Hooke’s drawing of cork, from Micrographia.
Some organisms, such as the amoeba, are individual cells. Higher creatures, be they delphiniums, tigers or people, are huge assemblies of cells. At first it looked as though the crucial distinction between organisms was the number of cells they contained: one, or more. Single-celled organisms were simpler than many-celled ones. But when microscopists discovered how to see the different bits and pieces that made up a cell, they realised that there was a more fundamental difference. Some single-celled organisms – for instance, bacteria – were very different from other single-celled organisms, such as the amoeba. Most many-celled organisms belonged in the same category as some of the single-celled organisms, and the others were not so much organisms as colonies.
The fundamental distinction, in fact, is between prokaryotes and eukaryotes – two of the three ‘domains’ into which life is now classified. (The third domain is archaea, primitive single-celled creatures that used to be grouped with prokaryotes.) Eukaryote cells possess a nucleus; prokaryote cells don’t. Bacteria are prokaryotes; amoebas and tigers are eukaryotes. Why so much fuss about the nucleus? Because it affects how the cell reproduces. All cells multiply by dividing: a single ‘mother’ cell splits, forming two ‘daughter’ cells. But prokaryotes do this in a much simpler manner than eukaryotes.
When a cell divides, it splits into two pieces, each roughly half the size. Each piece is a new cell, a sort of copy of the original, and if necessary it can grow bigger. But reproduction must copy not just the form of the cell but the genetic information hidden inside it, because the genetics controls many of the processes that keep a cell alive. The genes are collected together in regions of the cell known as chromosomes, ‘coloured bodies’, a term that reflects their discovery when parts of the cell were selectively stained using dyes. When the cell divides, the chromosomes must somehow be copied, with one copy going to each daughter cell. This copying process is very different in prokaryotes and eukaryotes.
A prokaryote cell has a number of components (see Figure 4). Most of them are enclosed in an envelope – a bag that holds vital parts together. This has two layers: an outer cell wall and an inner membrane. The envelope is fairly rigid, so it helps the cell to maintain its shape. It is not totally impervious: some things are allowed in, some are allowed out. Its job is to control what goes each way. The outside is usually, but not always, decorated with structures that aid movement (flagella, plural of flagellum) and communication (pili, plural of pilus). A flagellum is a tail-like protuberance which can spin, propelling the cell through the surrounding fluid medium. A pilus is a hair-like appendage, and cells can link their pili together, providing a communication channel between their interiors.
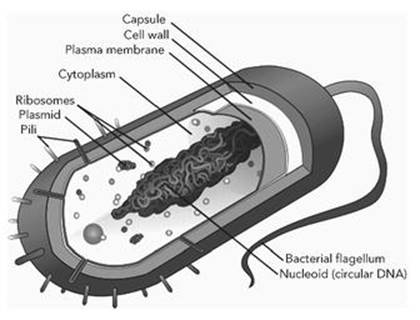
Fig 4 Prokaryote cell.
The region inside the cell envelope contains various special components, among them ribosomes, which make proteins, and the genetic material, which among other things specifies the structure of those proteins. The genetic material, which we now know is DNA, is almost always a long, closed loop, folded into a complicated tangle and attached to the membrane. There may also be free-floating loops of DNA, called plasmids. These permit ‘bacterial sex’, in which DNA is exchanged via the pili.
Eukaryote cells are more complex than prokaryotes, and usually larger, 10 – 15 times as wide and enclosing a thousand times the volume (see Figure 5). There is a cell membrane, but not always a cell wall. In place of flagella and pili there may be cilia, which wave from side to side, helping the cell to move. The most important difference is the genetic material. In a eukaryote cell, most of this is segregated inside a nucleus, which has its own membrane. It also consists of DNA, but now this molecule consists of long strands, not closed loops. The strands are organised by being wound round bobbin-like molecules called histones, and each strand forms a separate chromosome.
Eukaryote cells contain several other structures, known as organelles (‘little organs’). Among them are ribosomes, which again make proteins, and mitochondria (plural of mitochondrion), which manufacture a molecule called adenosine triphosphate (ATP), which in turn generates the cell’s energy.
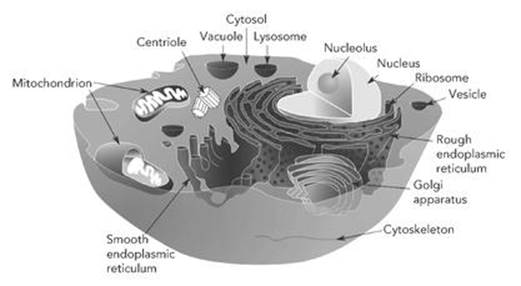
Fig 5 Eukaryote cell, with its organelles labelled.
The ability of cells to move, when witnessed through a microscope, seems almost miraculous. They seem to know where they’re going. However, we know enough about cellular movement to penetrate the apparent miracle and understand a little of what makes it tick. It depends on an organelle that controls the cell’s shape; suitable changes in shape result in movement. The shape is maintained and changed using a kind of skeleton formed from long tubular molecules. These tubes can grow or come to pieces as required, and they are manufactured by another organelle, the centrosome.
The main agent of cellular movement is the cytoskeleton, a web of protein scaffolding inside the cell. It is built in part from microtubules – long, thin tubes made from a protein called tubulin. Tubulin occurs in two very similar but distinct forms, alpha- and beta-tubulin. The structure of a microtubule is like a chessboard rolled into a tube: the ‘black’ squares are alpha-tubulin and the white ones are beta-tubulin. Dynamically, this structure is unstable – it is like a cylindrical brick chimney in which successive rows of bricks fit precisely on top of one another instead of being staggered.
Why does nature make such an important item as a microtubule in such an unstable way? Because the ‘cleavage lines’, where the structure is weak, are useful. Microtubules can grow longer by adding another layer of protein bricks. But they can also shorten, splitting apart at the seams like a banana being peeled. Experiments show that they shorten about ten times as rapidly as they grow, and mathematical models of the forces that act between molecules and atoms support this observation. So the cell can go ‘fishing’ for interesting things using tubulin rods, pushing them out at random to see what they find, and collapsing them if they don’t find anything. A cell moves by demolishing and rebuilding its own skeleton. It all boils down to the dynamics of a tiny molecular machine.
The construction and demolition of microtubules are controlled by chemical signals, many of which have an environmental origin. If the cell receives signals associated with food, it tears down its scaffolding on the side opposite the food, builds more of it on the side facing the food, and so inches its way foodwards.
Microtubules are produced by an organelle called the centrosome, first described by Theodor Boveri and Edouard van Beneden in 1887. When a cell divides, its chromosomes must replicate, and this process centres around a structure called the mitotic spindle. The chromosomes line up around the ‘equator’ of the mitotic spindle and subsequently migrate to its ‘poles’. Through their microscopes, Boveri and van Beneden spotted a tiny dot at each pole of the mitotic spindle – a centrosome (see Figure 14, p. 87). A single cell has one centrosome, close to its nucleus. When the cell divides, the centrosome splits into two pieces which move apart. The mitotic spindle forms between them. Then the centrosomes pull the cell into two parts by extruding microtubules, which they use as fishing rods to grab chromosomes and pull them into the required positions using special chemical motors.
The centrosome consists of two identical molecular machines, the centrioles. Each centriole is a bundle of twenty-seven microtubules, arranged symmetrically in nine sets of three, glued together with a slight twist. Two of these devices, arranged at right angles to each other, are surrounded by a fuzzy cloud of ‘pericentriolar material’ from which sprout numerous tubulin fishing-rods. This elegant molecular machine also organises the production of new microtubules.
A combination of mathematical modelling and biochemistry has recently revealed yet another role for tubulin in a cell. Small molecules can diffuse through the cell unaided, but large, biologically important ones may not get to where they are needed if left to their own devices. A protein molecule known as kinesin ‘walks’ along the tubulin rods on little molecular legs,1 carrying vital molecules across the cell. So a cell is not just a bag of chemicals – it is more like a highly automated factory.
In prokaryotes and single-celled eukaryotes, the organism is a cell, so division of the cell constitutes reproduction of the organism. In many-celled higher life forms, including all the animals and plants familiar from everyday life and David Attenborough’s television programmes, a lot more has to happen before the adult organism reproduces. In sexual organisms – the majority – male cells divide in a special way to produce sperm, and female cells similarly produce eggs. (I will describe this briefly later, when we have more background: both sperm and egg have half the normal amount of genetic material found in a cell.) These two types of specialist ‘halfcell’ are then brought together, the sperm fertilises the egg, and the two together form one conventional cell.
Once fertilised, the egg undergoes a complicated but organised pattern of development. In mammals, this takes it through a series of stages – embryo, fetus – leading to the point at which it emerges from its mother into the outside world. It then continues to develop through its juvenile stage until it becomes an adult. It is the same in birds and reptiles, except that for ‘mother’ you should read ‘egg’. Other types of organism go through corresponding changes: for example, a frog develops through the tadpole stage and eventually turns into a miniature adult. Only at that point can the original adult organism be said to have reproduced.
Development is perhaps the most complex part of biology, because that is the stage at which we are forced to contemplate not just some isolated part of a living creature, but the whole creature. What we know about development is enormous, but what we don’t know is far bigger. We know, in astonishing detail, how innumerable organisms develop – dogs, cats, dogfish, catfish, pigeons, spiders, marigolds, lizards, sea urchins, fruit flies, tiny nematode worms . . . But we have far less understanding of the processes that control development.
The gist of it seems to be that the fertilised egg divides repeatedly, providing an ever-increasing number of cells, and that the organism’s genetic material somehow orchestrates the patterns by which these cells grow, move, specialise to perform particular tasks and even die. Often the way nature makes a complicated structure involves using some cells as temporary scaffolding, and killing them off when they are no longer needed.
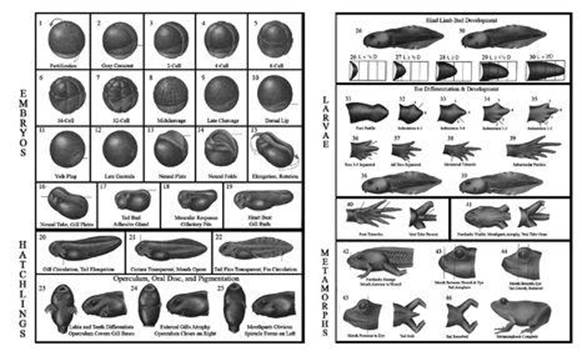
Fig 6 Early stages in development of the frog Bufo valliceps.
The early stages of development are similar in most of the higher animals; those of the frog are shown in Figure 6. The fertilised egg divides repeatedly without any change in the total size of all the cells, so the cells themselves become smaller and smaller. This process is known as cleavage, and it leads to a blastula, a hollow sphere of tiny cells. In many species this sphere is filled with fluid or yolk, but in mammals it contains another mass of cells, called the blastocyst.
Various layers of different cells appear; then a key step, known as gastrulation, occurs, and the entire ball of cells folds in on itself to create something more closely resembling a bag, or a tube with a hole at one end. (I’ll describe a mathematical model of this process at the end of Chapter 13, which takes the cellular structure into account.) At this point the organism acquires an inside as well as an outside – so to speak. The internal organs can now form.
Next, we observe the beginnings of the nervous system. Two parallel ridges appear on the outside of the developing embryo, creating a groove between them; this is called the neural groove. It closes up into a tube, the neural tube, which later develops into the spinal cord and the nervous system. Another tube forms, which later becomes the digestive system, from mouth via stomach and intestines to anus. A rudimentary brain starts to appear ... and so on, and so on, and so on.
Biochemistry alone cannot explain the complex changes of form that accompany development. We also have to take account of the physical properties of cells, such as how sticky they are, how they migrate from one region of the embryo to another, how new cells are born and how existing cells die. The appearance of stickiness in cells paved the way for the evolution of many-celled organisms; without it, they’d fall apart.
Development includes the deliberate destruction of cells that are employed as ‘scaffolding’ while some structure is forming, and are then destroyed once it has been made. This process is known as apoptosis, or programmed cell death. For example, an embryonic chicken’s limbs develop from limb buds. At first the bud is just a single, featureless rounded shape, but after a time it splits into separate finger-like protrusions. This splitting is not just caused by separate protuberances growing: in the regions between them, cells die, much as a seamstress may make gloves by cutting away material between the fingers rather than sewing separate fingershaped pieces of cloth together. Mathematical models have added to our understanding of limb growth, the shape of flies’ wings, the tentacles of the tiny Hydra, and many other developmental puzzles.
Development is not just about molecular structure: its most important feature is shape. An organism cannot function effectively if its organs, limbs and body are the wrong shape. Biologists have learned a great deal about the changes that occur as an embryo develops. In insects, for example, large-scale structures such as legs and antennae develop from small regions of cells called imaginal discs. Experiments show that the growth and movement of these cells are controlled in part by specific genes, known as Hox genes. A mutation in one of these genes can, for instance, cause an antenna to form where a leg ought to be. Other genetic errors can create legs where there ought to be antennae.
Development involves an intricate interaction between genetics and physical processes of growth, movement and death. We are only just beginning to understand such processes, which pose a fascinating challenge for biologists, physicists, chemists and mathematicians.