Homework Helpers: Physics
5 Electric Charges, Forces, and Fields
I remember going with my brother and sisters to visit my grandmother in her apartment that had a long, carpeted hallway. My siblings and I would spend much of our time shuffling up and down the hallway, building up static charges on our bodies, only to discharge these charges onto each other. A spark would be visible and a crackle would be audible as we shocked each of our victims. We never grew tired of the game, and to this day, I am still fascinated by the electric discharges that can be produced when an excess of electrons are built up on an object. In this chapter, we will explore some of the aspects of static electricity.
Lesson 5–1: Electric Charges
What people often refer to as static electricity or static charge is a buildup of charge on an object. How do objects obtain charges, and what do people mean when they say that there are two types of charge?
If you ever played with bar magnets, then you know that you can put two like poles together, say north to north, and they will repel each other. You can also put two unlike poles together, one north and one south, and they will attract each other. Similarly, some charged objects repel each other and others attract each other. This evidence suggests that there are two different types of charges.
When you take clothes out of the dryer and find a sock stuck to a sweater, we call this static cling. Static cling results when one item of clothing has a positive charge and the other has a negative charge. As is the case with magnets, unlike objects attract. But how does a sock obtain a charge?
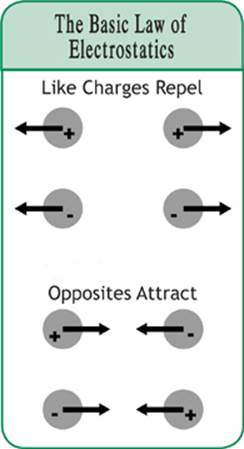
Figure 5.1
Thinking back to your study of chemistry, you should recall two types of charged subatomic particles, the proton and the electron, with a positive charge and a negative charge, respectively. Atoms can gain or lose electrons to become ions, or charged atoms. A negative ion is an atom that has gained one or more extra electrons. A positive ion is an atom that has lost one or more electrons.
When we say that an atom, or any object, has lost an electron, we don”t mean that it was destroyed. Electrons travel from atom to atom, or object to object, but they aren”t created or destroyed in the process of normal charging. In most of the cases that we will discuss in this chapter, charging is accomplished by getting some electrons to leave one object and go to another, leaving the first object with a deficiency of electrons and a positive charge, and the second object with an excess of electrons and a negative charge.
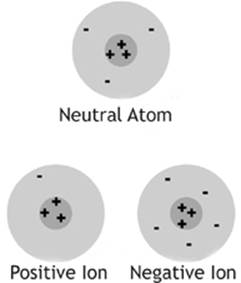
Figure 5.2
The electron is thought to have the smallest amount of negative charge possible. The proton is thought to have the smallest possible amount of positive charge. These elementary charges (e) are equal in magnitude, but opposite in type. In physics we represent charge with the letter q. We measure charges in units called coulombs (C). One coulomb actually represents a relatively large charge, so the elementary charge, measured in coulombs, is incredibly small.
Elementary Charges
The charge on 1 electron (e–) = −1.60 × 10–19 C.
The charge on 1 proton (p+) = +1.60 × 10–19 C.
When you studied chemistry, you probably represented ions with superscripts, indicating the number of electrons it had gained or lost. For example, the sulfide ion (S2–) has two extra electrons, giving it a net charge of -2. If you were asked to indicate the charge on this ion in coulombs, you would multiply the number of excess electrons (n) by the charge on one electron (e), as shown here:
![]()
If an ion is missing electrons, it has more protons then electrons, giving it a net positive charge. If you were asked to give the charge of a positive ion in coulombs, you multiply the number of excess protons by the charge on one proton.
Example 1
Determine the charge in coulombs of an ion of aluminum, Al3+.
Given: Number of extra protons (n) = 3
Charge on one proton (e) = +1.60 × 10–19 C
Find: q
![]()
As with the case with atoms, neutral macroscopic objects contain the same number of protons and electrons, and have a net charge of zero. Charged objects have an unequal number of protons and electrons. If they have more electrons than protons, they have a net negative charge. If they have fewer electrons than protons, then they have a net positive charge.
Example 2
A woman walks across a carpet and obtains a charge of −4.56 × 10–16 C. How many excess electrons does this represent?
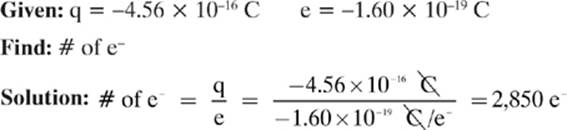
Remember: If the woman took 2,850 electrons from the carpet, giving her a net charge of –4.56 × 10–16 C, the carpet would have a deficiency of 2,850 electrons, giving it a net charge of +4.56 × 10–16 C, so the total charge would be conserved.
This brings us back to the sweater and sock example from the beginning of the lesson. As clothes come in contact with each other in the dryer, some items give up some of their electrons and obtain a net positive charge, whereas others take on some additional electrons, giving them a net negative charge. Consequently, oppositely charged items can stick together because of the electrostatic force of attraction between them.
Lesson 5–1 Review
1. A/an ________________ is positively charged particle commonly found in the nucleus of an atom.
2. A/an ________________ is an atom that has gained one or more electrons.
3. An oxide ion (O2–) has gained two additional electrons. What is the charge on such an ion, in coulombs?