Homework Helpers: Physics
9 Heat and Thermodynamics
I”m sure that you have heard of a method for starting a fire that involves rubbing two sticks together. In this process, the kinetic energy that your hands transfer to the sticks will be converted into thermal energy because of the friction between the two sticks. As more and more kinetic energy is converted to thermal energy, the temperature of the two sticks continues to rises. Eventually, the sticks may get hot enough to ignite some kindling. In this chapter we will study heat, temperature, and the law of thermal energy.
Lesson 9–1: Heat and Temperature
Heat and temperature are two quantities that can be easily confused. Imagine cooking a very large vat of chicken soup on the stove. Let”s suppose you heat the soup until it is 95°C, quite hot. You take a tablespoon and scoop out a spoonful of soup to taste. As you remove the spoonful of soup from the vat, it has the same temperature (95°C) as the larger sample. Unfortunately, as you bring the soup toward your mouth to taste it, the spoon slips from you hand, pouring its contents on your bare foot. A spoonful of 95°C soup hitting your foot hurts, but not as bad as it would if you accidentally spilled the entire vat of 95°C soup on your foot. If both the spoonful and the vat full of soup have the same temperature, why would the larger sample cause more damage if it came in contact with your skin? The answer to the question lies in the difference between temperature and heat.
Temperature is defined as a measure of the average kinetic energy of the particles of a substance. The keyword in this definition is average. You can have a very small sample and still have a high average, just as you could have a very large sample and still have a very small average. The molecules of soup in our spoon had the same average kinetic energy as the molecules in the vat, but the vat contained many more molecules.
Let”s suppose two different classes took part in a fund-raiser. Class A, containing 14 students, collected a total of $280. Class B, consisting of 30 students, collected $600. The average amount of money collected by the students in each class would be the same, despite the fact that class B raised more total money.

There is another important reason to remember that temperature represents the average kinetic energy of the particles of a substance. In our fund-raising example, there could have been students who raised much more or much less than the class average. One student, for example, may have raised $120 while another raised $2. The class average doesn”t tell us how much money each specific student raised. In much the same way, the temperature of a sample doesn”t tell us the kinetic energy of a specific molecule. In any sample, there will be some molecules with greater-than-average kinetic energy and some with less than the average kinetic energy. This helps us understand how some molecules can evaporate, going from a liquid to gas phase, in a sample of a liquid with a temperature far below its boiling point. It also helps us to understand why heat is lost from an object or sample when evaporation occurs. The evaporating molecules represent molecules of higher than average kinetic energy, and when they leave the sample, the average kinetic energy (temperature) goes down.
Temperature Conversions
The Kelvin scale is the SI scale for temperature, and it is based on the concept of absolute zero. Absolute zero is theoretically the lowest temperature that an object can reach. At this temperature, the kinetic energy of a molecule will be zero. Although the kelvin is the SI unit for temperature, you are still likely to encounter the Celsius scale. The following formulas are used to convert between Celsius and kelvin.
K = °C + 273
°C = K – 273
Example 1
Convert 25°C to kelvin.
To convert Celsius to kelvin, simply add 273, and drop the degree (°) symbol.
Solution: 25°C = (25 + 273) = 298 K
The Fahrenheit scale is still used in the United States, but it is considered outdated almost everywhere else. If you need to convert between Celsius and Fahrenheit, use the following formulas.
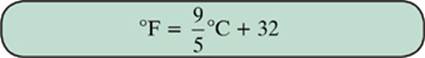
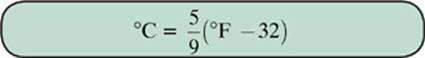
Example 2
Water boils at 100°C. What is the equivalent temperature on the Fahrenheit scale?
![]()
Heat is a measure of the thermal energy that is transferred from one body to another. In our soup example, the vat of soup would transfer more heat to your foot than the spoonful because the larger sample contains a greater amount of thermal energy. Like other forms of energy, heat is measured in the SI-derived units called joules (J). Another unit of energy, calories (cal), is still often used to measure heat transfer. One calorie is approximately equal to 4.19 joules.
1 cal = 4.19 J
When calories are discussed with reference to food, they really refer to food calories. One food calorie is equal to 1,000 calories, or 4,190 J.
Lesson 9–1 Review
1. ________________ is a measure of the average kinetic energy of the particles of a substance.
2. Convert 34.0° Celsius to kelvin.
3. Convert 76° Fahrenheit to Celsius.