Homework Helpers: Physics
9 Heat and Thermodynamics
Lesson 9–3: Thermal Expansion
When you studied chemistry, you probably learned that one of the unusual properties of water is that within a certain range (0°C to 4°C) of temperatures, it gets more dense as its temperature increases. As a solid, water is less dense, which allows ice to float on liquid water. Most substances tend to expand to some extent when they are heated. This is to be expected if you understand the definition of temperature that we went over in our last lesson. Molecules at greater temperatures have greater kinetic energy. When fast-moving molecules crash into each other, they bounce away to a greater degree than slow-moving molecules would, just as fast-moving bumper cars would bounce back more from a head-on collision than slow-moving ones would. This is what causes the size of objects to increase slightly as they get hotter, which is called thermal expansion, and the density of such materials to decrease.
For this lesson, we will only concern ourselves with linear expansion, which basically involves an increase in the length of an object as its temperature increases. As you can surmise from our example of water, not all materials show the same degree of expansion as their temperatures change. The degree to which an object expands as its temperature rises depends on the material from which the object is made. The formula for linear expansion can be used to calculate the change in length an object will experience, based on the original length of the object, the change in temperature of the object, and the coefficient of linear expansion for the material from which the object is made.
Linear Expansion
ΔL = αLiΔT
change in length =
coefficient of linear expansion × initial length × change in temperature
Example 1
A bar of a certain metal has an initial length of 1.000 m at 10.0°C. When the bar is heated to 90.0°C, the length of the bar is carefully measured to be 1.002 m. What is the coefficient of linear expansion for this metal?
Given: Li = 1.000 m Lf = 1.002 m Ti = 10.0°C Tf = 90.0°C
Find: α

Example 2
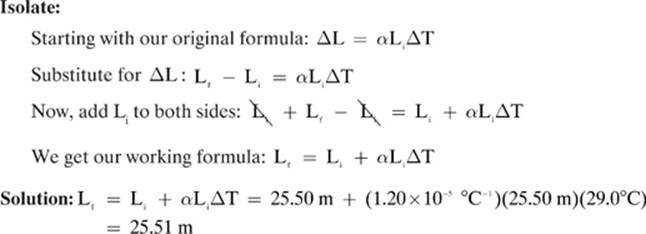
Concrete has a coefficient of linear expansion of approximately 1.20 × 10–5 °C–1. If a stretch of concrete has a length of 25.5 m at 11.0°C, what will be the length of the stretch at 40.0°C?
Given: α = 1.20 × 10—5 °C–1 Li = 25.50 m Ti = 11.0°C Tf = 40.0 °C
Find: Lf
As you might imagine, when the length of a solid object expands, its width and height are likely to expand as well. Sometimes you will want to be able to calculate the change in the volume of a substance as its temperature changes.
For most solid objects, the change in the volume due to a change in temperature can be approximated with the formula:
ΔV = 3αViΔT
When you are asked to calculate the change in volume for a liquid, you will likely be given a coefficient of volume expansion, represented by the Greek letter beta (β). The coefficient will be used in the formula for volume expansion.
Volume Expansion
ΔV = βViΔT
Change in volume =
coefficient of volume expansion × initial volume × change in temperature
Example 3
Mercury has a coefficient of volume expansion (β) of 1.6 × 10–4 °C–1. If the temperature of a sample of mercury with an initial volume of 2.50 × 10–2 m3 changes from 5.00°C to 34.0°C, what will be the final volume of the sample?
Given: β = 1.6 × 10–4 °C–1 ΔT = T f – Ti = 34.0°C – 5.00°C = 29.0°C Vi = 2.50 × 10–2 m3
Find: Vf
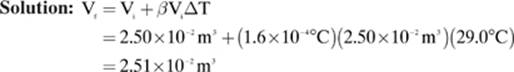
Lesson 9–3 Review
1. Calculate the coefficient of volume expansion of a specific liquid if a pure sample has a volume of 2.343 × 10–5 m3 at 2.00°C and a volume of 2.397 × 10–5 m3 at 45.0°C.
2. A block of a certain material has an initial length of 9.452 × 10–1 m, at 6.00°C. When the block is heated to 64.0°C, its length of the bar is carefully measured to be 9.478 × 10–1 m. What is the coefficient of linear expansion for this metal?
3. Gasoline has a coefficient of volume expansion (β) of 9.5 × 10–4 °C–1. If the temperature of a sample of gasoline with an initial volume of 3.547 × 10–1 m3 changes from 8.00°C to 57.0°C, what will be the final volume of the sample?