Homework Helpers: Physics
9 Heat and Thermodynamics
Answer Key
The actual answers will be shown in brackets, followed by an explanation. If you don”t understand an explanation that is given in this section, you may want to go back and review the lesson that the question came from.
Lesson 9–1 Review
1. [Temperature]—Remember, the keyword in this def, inition is average.
2. ![]()
3. ![]()
Lesson 9–2 Review
1. [Specific heat or specific heat capacity]—Objects with low specific heat capacities will heat up quicker.
2. 
3. 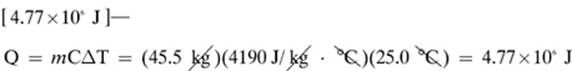
Lesson 9–3 Review
1. [ 5.36 × 10-4 °C-1—
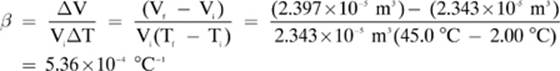
2. [4.74 × 10–5 °C–1]—
Given: Li = 9.452 × 10–1 m Lf = 9.478 × 10–1 m Ti = 6.00°C Tf = 64.0 °C
Find: α
Solution:
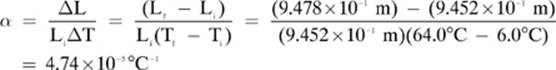
3. [3.7 × 10–1 m3]—
Given: β = 9.5 × 10-4 °C-1 ΔT = Tf – Ti = 57.0°C – 8.00°C = 49.0°C Vi = 3.547 × 10-1 m3
Find: Vf
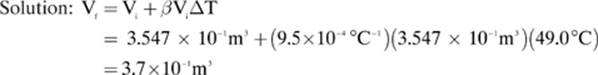
Lesson 9–4 Review
1. [Charles”s law]—The formula for Charles”s law is V T V
![]()
2. [3.0 atm]—Because our solution to this problem will not involve one of the constants, we can use the units that came with the problem, with the exception for the temperature, which must always be converted to kelvin.
Convert: T1 = 24.0°C = (24.0 + 273) = 297 K
T2 = 49.0°C = (49.0 + 273) = 332 K
Given: V1 = 6.65 cm3 T1 = 297 K P1 = 2.0 atm V2 = 5.00 cm3 T2 = 332 K
Find: P2
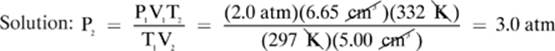
3. [9.92 × 1025]—When you want to solve for the number of particles in a sample, we use the formula PV = NkB T, which contains the Boltzmann constant (kB), with a value of 1.38 × 10–23 J/K. Remember: As with our other gas law constant, keep in mind that 1 J = (1 × 10–3 m3) · Pa. This means that we can use the units for volume and pressure that came with our problem. We only need to convert the temperature to kelvin.
Convert: T = 25.0°C = (25.0 + 273) = 298 K
Given: P = 3.4 × 105 Pa V = 1.20 m3 T = 298 K
kB = 1.38 × 10–23 J/K
Find: N
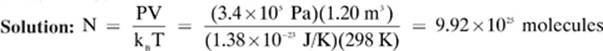
Lesson 9–5 Review
1. [8.45 × 103 J ]—W = PΔV = (2.45 × 105 Pa)(0.0345 m3) = 8.45 × 103 J
2. [260 J]—Given: Q = 340 J W = 80 J
Find: ΔU
Solution: ΔU = Q – W = 340 J – 80 J = 260 J
3. [isobaric]—Simply think of a “barometer”, which is an instrument that is used to measure gas pressure.
Chapter 9 Examination
1. [b. kelvin]—Remember to do all gas law calculations in kelvin, so that you don”t end up with negative values for quantities, such as volume, that have no negative values.
2. [d. Boyle”s law]—The formula for Boyle”s law shows this relationship. P1 V1 = P2 V2
3. [i. specific heat]—Specific heat is also sometimes called “specific heat capacity.”
4. [a. absolute zero]—Although this temperature has never been achieved, it can be calculated.
5. [h. pressure]—Pressure is often measured in N/m2.
6. [b. The pressure would increase by a factor of three.]—According to the ideal gas law, the pressure and temperature of the gas will vary directly, so tripling the temperature at constant volume will triple the pressure.
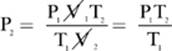
7. [d. volume]—Think of “metric” and “meter.”
8. [a. radiation]—All heat transferred by electromagnetic waves represents radiation.
9. [315 J]— ΔU = Q – W = 235 J – (–80 J) = 315 J
10. ![]()
11. ![]()
12. [158 °C]—°C = K – 273 = 431 – 273 = 158°C
13. 
14. 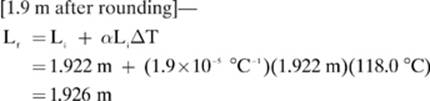
15. [2.75 × 104 J]—W = PΔV = (1.55 × 105 Pa)(0.1777 m3) = 2.75 × 104 J
16. 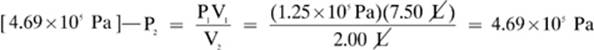
17. 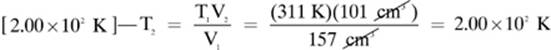
18. 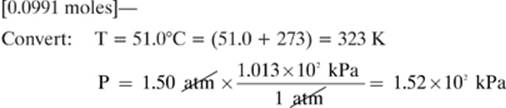
Given: V = 1.75 dm3 T = 323 K P = 1.52 × 102 kPa R = 8.31 dm3 × kPa/mol × K
Find: n
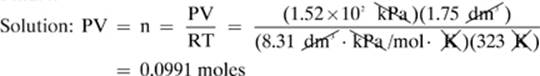
19. [542 m/s]—
Convert: T = 57.0 °C = (57.0 + 273) = 330. K
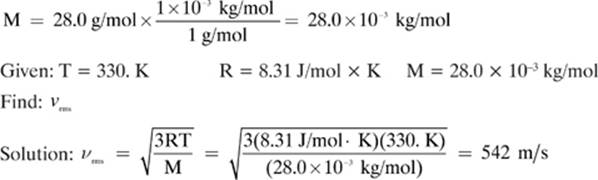
20. [–163 000 calories]—
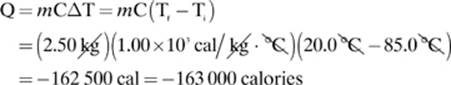
after rounding to three significant digits. The negative sign simply indicates that heat was lost.