Homework Helpers: Physics
10 Nuclear Physics
In most chemistry books, the lessons on atomic structure are found near the beginning. Yet, in most physics books, they are found near the end. Perhaps this is because students need to understand many of the other topics in physics before they can hope to understand the complex structure of the atom. Another reason is because this chapter marks the beginning of a newer branch of study. Up until now, we have been studying mainly macroscopic objects, which are big enough to see with the naked eye, and they appear to follow the laws of Newtonian mechanics. The microscopic, subatomic particles that we study in this chapter don”t appear to follow all of those laws. A new set of rules that allow for strange observations, such as mass being transformed into energy, apply here.
Lesson 10-1: Structure of the Atom
If you studied chemistry, then you probably know the basics of atomic structure. We reviewed some of the subatomic particles earlier in this book, but now we will go over the structure of the atom in a more formal way.
There are three main types of subatomic particles:
1. The proton is a positively charged subatomic particle normally found in the nucleus of the atom. Each proton has a charge of +1.60 × 10–19 C, and a mass of approximately 1.672 65 × 10–27 kg, or 1.007 825 atomic mass units (u). Because protons are normally found in the nucleus of the atom, they, along with neutrons, are sometimes called nucleons. Protons are also classified as hadrons, because they are made up of smaller particles called quarks. The number of protons in the nucleus of an atom is called the atomic number (Z), or nuclear charge. The number of protons in the nucleus of an atom determines its identity. For example, every hydrogen atom has only one proton in its nucleus.
2. The neutron is a neutrally charged subatomic particle that is also typically found in the nucleus. The neutron has a neutral charge and a mass of approximately 1.674 95 × 10–27 kg, or 1.008 665 atomic mass units (u). Neutrons and protons are sometimes collectively called nucleons because they make up the nucleus of atoms. They are also examples of hadrons because they are made up of smaller particles called quarks. Atoms of a particular element that have different numbers of neutrons in their nucleus are called isotopes. For example, the isotope of hydrogen called protium has one proton and zero neutrons in its nucleus, and the isotope of hydrogen called deuterium has one proton and one neutron in its nucleus.
3. Electrons are not found in the nucleus of the atom, so please don”t call them nucleons. They are found in an area surrounding the nucleus called the electron cloud. The mass of each electron is only about 9.109 × 10–31 kg, or 5.49 × 10–4 u. Electrons are thought to be truly “elementary particles” belonging to a class called leptons. An atom can lose one or more electrons to become a positive ion, or it can gain one or more additional electrons to become a negative ion.
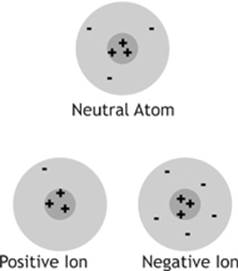
Figure 10.1
Example 1
The mass of a proton (mp = 1.672 65 × 10-27 kg) and the mass of an electron (me = 9.109 × 10-31 kg) may not seem all that different to you, but the mass of the electron is much smaller. In fact, the mass of the electron is usually considered insignificant, so much so that the mass of the electrons are often not even taken into account when calculating the mass of a particular atom. How many electrons would you need to add together to approximate the mass of a proton?
We can solve this by dividing the mass of a proton by the mass of an electron.
Solution:
![]()
So, it would take about 1,836 electrons to equal the mass of one proton. This illustrates why the mass of an electron is often overlooked. This can also help you understand why the electron exhibits wave-like properties that can be measured, as we will discuss shortly.
The tiny mass of an electron can also help you understand one reason why the more predictable planetary model has given way to the current quantum-mechanical model of the atom. In Chapter 8 we discussed the so-called photoelectric effect, where light shining on the surface of a piece of metal can cause electrons to be ejected from the metal atoms. German physicist Werner Heisenberg pointed out that in order to locate the position of an electron, we would have to bounce photons of light off it, and that would surely change the momentum of the electron.
The Heisenberg Uncertainty Principle
It is impossible to determine accurately both the momentum and position of an electron simultaneously.
According to our current model of the atom, we can never truly know both the accurate position and momentum of an electron. We can only discuss the location of the electrons in the electron cloud in terms of probability. The likelihood of a particular electron to be found in a given area is the closest we can hope to determine.
Lesson 10–1 Review
1. A _______________ is a positively charged particle commonly found in the nucleus of the atom.
2. What part of the atom contains all of the positive charge and essentially all of the mass?
3. Where are the electrons of an atom commonly found?