SAT Physics Subject Test
Chapter 16 Modern Physics
WAVE–PARTICLE DUALITY
Light and other electromagnetic waves exhibit wave-like characteristics through interference and diffraction. However, as we saw in the photoelectric effect, light also behaves as if its energy were granular, composed of particles. This is wave–particle duality: Electromagnetic radiation propagates like a wave but exchanges energy like a particle.
Since an electromagnetic wave can behave like a particle, can a particle of matter behave like a wave? In 1923, the French physicist Louis de Broglie proposed that the answer is yes. His conjecture, which has since been supported by experiment, is that a particle of mass m and speed v—and thus with linear momentum p = mv—has an associated wavelength, which is called its de Broglie wavelength.
λ = ![]()
Particles in motion can display wave characteristics, and behave as if they had a wavelength λ = h/p.
Since the value of h is so small, ordinary macroscopic objects do not display wave-like behavior. For example, a baseball (mass = 0.15 kg) thrown at a speed of 40 m/s has a de Broglie wavelength of
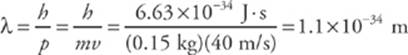
This is much too small to measure. However, with subatomic particles, the wave nature is clearly evident.
![]()
Questions 6-7
6. Name or describe an experiment that demonstrates that light behaves like a wave.
7. Name or describe an experiment that demonstrates that light behaves like a particle.
Here”s How to Crack It
6. Young”s double-slit interference experiment shows that light behaves like a wave. Interference is a characteristic of waves, not of particles.
7. The photoelectric effect shows that light behaves like a particle, where the energy of the light is absorbed as photons: individual “particles” of light energy.
![]()