SAT Physics Subject Test
Chapter 16 Modern Physics
NUCLEAR PHYSICS
The nucleus of the atom is composed of particles called protons and neutrons, which are collectively called nucleons. The number of protons in a given nucleus is called the atom”s atomic number, or Z, and the number of neutrons (the neutron number) is denoted N. The total number of nucleons, Z + N, is called the mass number (or nucleon number), and is denoted A. The number of protons in the nucleus of an atom defines the element. For example, the element chlorine (abbreviated Cl) is characterized by the fact that the nucleus of every chlorine atom contains 17 protons, so the atomic number of chlorine is 17; however, different chlorine atoms may contain different numbers of neutrons. In fact, about three-fourths of all naturally occurring chlorine atoms have 18 neutrons in their nuclei (mass number = 35), and most of the remaining one-fourth contain 20 neutrons (mass number = 37). Nuclei that contain the same numbers of protons but different numbers of neutrons are called isotopes.
The notation for a nuclide—the term for a nucleus with specific numbers of protons and neutrons—is to write Z and A, one above the other, before the chemical symbol of the element.
![]()
The isotopes of chlorine mentioned earlier would be written as follows:
![]()
![]()
8. How many protons and neutrons are contained in the nuclide ![]() ?
?
Here”s How to Crack It
The subscript (the atomic number, Z) gives the number of protons, which is 29. The superscript (the mass number, A) gives the total number of nucleons. Since A = 63 = Z + N, we find that N = 63 – 29 = 34.
![]()
![]()
9. The element neon (abbreviated Ne, atomic number 10) has several isotopes. The most abundant isotope contains 10 neutrons, and two others contain 11 and 12. Write symbols for these three nuclides.
Here”s How to Crack It
The mass numbers of these isotopes are 10 + 10 = 20, 10 + 11 = 21, and 10 + 12 = 22. So, we”d write them as follows:
![]()
Another common notation—which we also use—is to write the mass number after the name of the element. These three isotopes of neon would be written as neon-20, neon-21, and neon-22.
![]()
The Nuclear Force
Why wouldn”t any nucleus that has more than one proton be unstable? After all, protons are positively charged and would therefore experience a repulsive Coulomb force from each other. Why don”t these nuclei explode? And what holds neutrons—which have no electric charge—in the nucleus? These issues are resolved by the presence of another fundamental force, the strong nuclear force, which binds together neutrons and protons to form nuclei. Although the strength of the Coulomb force can be expressed by a simple mathematical formula (it”s inversely proportional to the square of their separatio`n), the nuclear force is much more complicated; no simple formula can be written for the strength of the nuclear force.
Binding Energy
The masses of the proton and neutron are listed below.
proton: mp = 1.6726 × 10–27 kg
neutron: mn = 1.6749 × 10–27 kg
Because these masses are so tiny, a much smaller mass unit is used. With the most abundant isotope of carbon (carbon-12) as a reference, the atomic mass unit (abbreviated amu or simply u) is defined as 1/12 the mass of a 12C atom. The conversion between kg and u is 1 u = 1.6605 10–27kg. In terms of atomic mass units
proton: mp = 1.00728 u
neutron: mn = 1.00867 u
Now consider the deuteron, the nucleus of deuterium, an isotope of hydrogen that contains 1 proton and 1 neutron. The mass of a deuteron is 2.01356 u, which is a little less than the sum of the individual masses of the proton and neutron. The difference between the mass of any bound nucleus and the sum of the masses of its constituent nucleons is called the mass defect, ∆m. In the case of the deuteron (symbolized d), the mass defect is
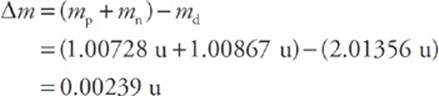
What happened to this missing mass? It was converted to energy when the deuteron was formed. It also represents the amount of energy needed to break the deuteron into a separate proton and neutron. Since this tells us how strongly the nucleus is bound, it is called the binding energy of the nucleus.
The conversion between mass and energy is given by Einstein”s mass–energy equivalence equation, E = mc2 (where c is the speed of light); the binding energy, EB, is equal to the mass defect, ∆m
EB = (∆m)c2
Using E = mc2, the energy equivalent of 1 atomic mass unit is about 931 MeV.
In terms of electronvolts, then, the binding energy of the deuteron is
![]()
Since the deuteron contains 2 nucleons, the binding energy per nucleon is
![]()
This is the lowest value of all nuclides. The highest, 8.8 MeV/nucleon, is for an isotope of nickel, 62Ni. Typically, when nuclei smaller than nickel are fused to form a single nucleus, the binding energy per nucleon increases, which tells us that energy is released in the process. On the other hand, when nuclei larger than nickel are split, binding energy per nucleon again increases, releasing energy.