SAT Physics Subject Test
Chapter 16 Modern Physics
RADIOACTIVITY
The stability of a nucleus depends on the ability of the nuclear force to balance the repulsive Coulomb forces between the protons. Many nuclides are ultimately unstable and will undergo spontaneous restructuring to become more stable. An unstable nucleus that will spontaneously change into a lower-energy configuration is said to be radioactive. Nuclei that are too large (A is too great) or ones in which the neutron-to-proton ratio is unfavorable are radioactive, and there are several different modes of radioactive decay. We”ll look at the most important ones: alpha decay,beta decay (three forms), and gamma decay.
Alpha Decay
When a nucleus undergoes alpha decay, it emits an alpha particle, which consists of two protons and two neutrons and is the same as the nucleus of a helium-4 atom. An alpha particle can be represented as
![]()
Very large nuclei can shed nucleons quickly by emitting one or more alpha particles, for example, radon-222 (![]() ) is radioactive and undergoes alpha decay.
) is radioactive and undergoes alpha decay.
![]()
This reaction illustrates two important features of any nuclear reaction.
(1) Mass number is conserved (in this case, 222 = 218 + 4).
(2) Charge is conserved (in this case, 86 = 84 + 2).
The decaying nuclide is known as the parent, and the resulting nuclide is known as the daughter. (Here, radon-222 is the parent nuclide and polonium-218 is the daughter.) Alpha decay decreases the mass number by 4 and the atomic number by 2. Therefore, alpha decay looks like the following:
![]()
Beta Decay
There are three subcategories of beta (β) decay, called β−, β+ and electron capture (EC).
β– Decay
When the neutron-to-proton ratio is too large, the nucleus undergoes β– decay, which is the most common form of beta decay. β– decay occurs when a neutron transforms into a proton and an electron, and the electron is ejected from the nucleus. The expelled electron is called a beta particle. The transformation of a neutron into a proton and an electron (and another particle, the electron-antineutrino, ![]() e) is caused by the action of the weak nuclear force, another of nature”s fundamental forces. A common example of a nuclide that undergoes β– decay is carbon-14, which is used to date archaeological artifacts.
e) is caused by the action of the weak nuclear force, another of nature”s fundamental forces. A common example of a nuclide that undergoes β– decay is carbon-14, which is used to date archaeological artifacts.
![]()
Notice how the ejected electron is written: The superscript is its nucleon number (which is zero), and the subscript is its charge. The reaction is balanced, since 14 = 14 + 0 and 6 = 7 + (–1).
β+ Decay
When the neutron-to-proton ratio is too small, the nucleus will undergo β+ decay. In this form of beta decay, a proton is transformed into a neutron and a positron, ![]() (the electron”s antiparticle), plus another particle, the electron-neutrino, ve, which are then both ejected from the nucleus. An example of a positron emitter is fluorine-17.
(the electron”s antiparticle), plus another particle, the electron-neutrino, ve, which are then both ejected from the nucleus. An example of a positron emitter is fluorine-17.
![]()
Electron Capture Another way in which a nucleus can increase its neutron-to-proton ratio is to capture an orbiting electron and then cause the transformation of a proton into a neutron. Beryllium-7 undergoes this process.
![]()
Gamma Decay
In each of the decay processes defined above, the daughter was a different element from the parent. Radon becomes polonium as a result of ± decay, carbon becomes nitrogen as a result of β– decay, fluorine becomes oxygen from β+ decay, and beryllium becomes lithium from electron capture. By contrast, gamma decay does not alter the identity of the nucleus; it just allows the nucleus to relax and shed energy. Imagine that potassium-42 undergoes β– decay to form calcium-42.
![]()
The asterisk indicates that the daughter calcium nucleus is left in a high-energy, excited state. For this excited nucleus to drop to its ground state, it must emit a photon of energy, a gamma ray, symbolized by γ.
![]()
![]()
10. What”s the daughter nucleus in each of the following radioactive decays?
(a) Strontium-90 ![]() ; β– decay
; β– decay
(b) Argon-37 ![]() ; electron capture
; electron capture
(c) Plutonium-239 ![]() ; alpha decay
; alpha decay
(d) Cobalt-58 ![]() ; β+ decay
; β+ decay
Here”s How to Crack It
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
![]()
Radioactive Decay Rates
Although it”s impossible to say precisely when a particular radioactive nuclide will decay, it is possible to predict the decay rates of a pure radioactive sample. As a radioactive sample disintegrates, the number of decays per second decreases, but the fraction of nuclei that decay per second—the decay constant—does not change. The decay constant is determined by the identity of the radioisotope. Boron-9 has a decay constant of 7.5 × 1017 s–1 (rapid), while uranium-238 has a decay constant of about 5 × 10–18 s–1 (slow).
The activity (A) of a radioactive sample is the number of disintegrations it undergoes per second; it decreases with time according to the equation
A = A0 eλt
where A0 is the activity at time t = 0 and λ is the decay constant (not to be confused with wavelength).
Activity is expressed in disintegrations per second: 1 disintegration per second is one becquerel (Bq). The greater the value of λ, the faster the sample decays. This equation also describes the number (N) of radioactive nuclei in a given sample, N = N0 eλt, or the mass (m) of the sample, m =m0 e−λt.
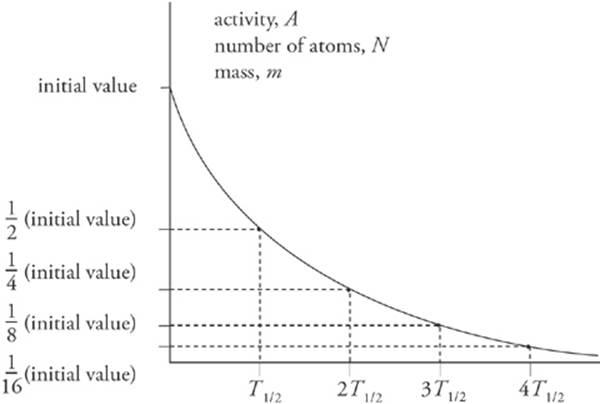
The most common way to indicate the rapidity with which radioactive samples decay is to give their half-life. Just as the name suggests, the half-life is the time required for half of a given sample to decay.
Half-life, T1/2, is inversely proportional to the decay constant, λ, and in terms of the half-life, the exponential decay of a sample”s mass (or activity) can be written as
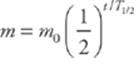
A sample”s activity or mass can be graphed as a function of time; the result is the exponential decay curve, which you should study carefully.
![]()
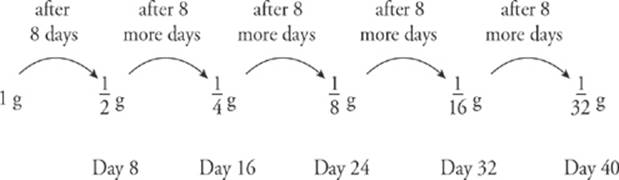
11. The half-life of iodine-131 (a β– emitter) is 8 days. If a sample of 131I has a mass of 1 gram, what will the mass be 40 days later?
Here”s How to Crack It
Every 8 days, the sample”s mass decreases by a factor of 2. We can illustrate the decay in the following diagram:
![]()
![]()
12. Home smoke detectors contain a small radioactive sample of americium-241 (![]() ), an alpha-particle emitter that has a half-life of 430 years. What is the daughter nucleus of the decay?
), an alpha-particle emitter that has a half-life of 430 years. What is the daughter nucleus of the decay?
Here”s How to Crack It
![]()
![]()