SAT Physics Subject Test
Chapter 16 Modern Physics
The subject matter of the previous chapters was developed in the seventeenth, eighteenth, and nineteenth centuries, but as we delve into the physics of the very small, we enter the twentieth century. Let”s first look at the structure of the atom, then travel into the nucleus itself. About 10 percent of the questions on the SAT Physics Subject Test will cover the field of modern physics.
THE RUTHERFORD MODEL OF THE ATOM
Around 1900, the atom was just considered a small bunch of positively charged “stuff” embedded with negatively charged electrons. This theoretical structure was known as the raisin pudding model; the pudding was the positively charged part of the atom and the raisins were the electrons. However, laboratory experiments in 1909 to 1911 led Ernest Rutherford to propose a radical revision of this model.
Rutherford fired alpha particles (α), which were known to be relatively massive and carry an electric charge of +2e, at an extremely thin sheet of gold foil. An alpha particle consists of two protons and two neutrons, tightly bound together. If the atom is really just a glob of positive charge dotted with tiny negative electrons, then the heavy alpha particles should sail right through the target atoms, with little deviation.
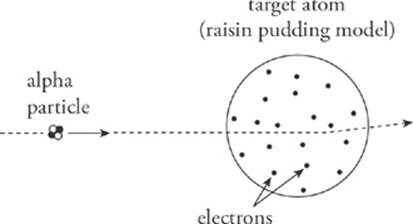
For the most part, this is what the experiments revealed. However, a small percentage of the alpha particles exhibited behavior that was completely unexpected: Some were deflected through very large angles (90° to 180°). This was explained by postulating that the positive charge of the atom was not spread throughout its volume, but was concentrated in a very tiny volume, at the atom”s center. Alpha particles that came close to this concentration of positive charge experienced a strong Coulombic repulsive force and were deflected through large angles.
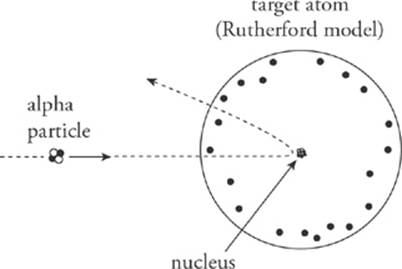
The tiny volume in which the positive charge is concentrated in an atom is known as the nucleus, and the nucleus is surrounded by a swarm of negatively charged electrons. This model is known as the Rutherford nuclear model.