Biology Premium, 2024: 5 Practice Tests + Comprehensive Review + Online Practice - Wuerth M. 2023
UNIT 1 Chemistry of Life
4 Macromolecules
Learning Objectives
In this chapter, you will learn:
➜Biological Macromolecules
➜Protein Structure
➜Nucleic Acids
Overview
Biological macromolecules form the basis for the structure and function of living organisms. This chapter reviews the basic reactions that form and break down these molecules. Then, the structure and function of carbohydrates, lipids, nucleic acids, and proteins are reviewed, with special attention given to the four levels of structure found in proteins.
Biological Macromolecules
The macromolecules necessary for life are primarily made of six elements: nitrogen, carbon, hydrogen, oxygen, phosphorus, and sulfur.
TIP
An easy mnemonic device to help you remember these elements is NCHOPS for Nitrogen, Carbon, Hydrogen, Oxygen, Phosphorus, Sulfur.
Carbon is the “backbone” of these molecules. Carbon has four valence electrons and is extremely versatile in the way it can bond to other atoms. It can form single, double, or even triple bonds. Carbon can also form linear, branched, or ring-type structures. Carbon is found in all types of macromolecules.
Oxygen and sulfur each have six valence electrons, and each of these elements typically forms two bonds. Oxygen is found in all types of macromolecules. Sulfur is typically found in proteins.
Nitrogen and phosphorus each have five valence electrons, and each of these elements typically forms three bonds. Nitrogen is found in nucleic acids and proteins. Phosphorus is found in nucleic acids and some lipids.
Hydrogen has one valence electron and forms a single bond. Hydrogen is found in all types of macromolecules. In fact, hydrogen atoms are so ubiquitous that often they are not drawn in molecular structures.
TIP
While you do not need to know the structures of specific molecules for the AP Biology exam, you do need to be familiar with the different types of structures and functions of macromolecules.
The structure and function of a macromolecule is determined by the types of monomers from which it is made and how the monomers are linked.
Making and Breaking Biological Macromolecules
Biological macromolecules are formed from building blocks (i.e., monomers) that are linked by dehydration synthesis to form larger molecules. These biological macromolecules are broken down by hydrolysis reactions. (See Figure 4.1.)
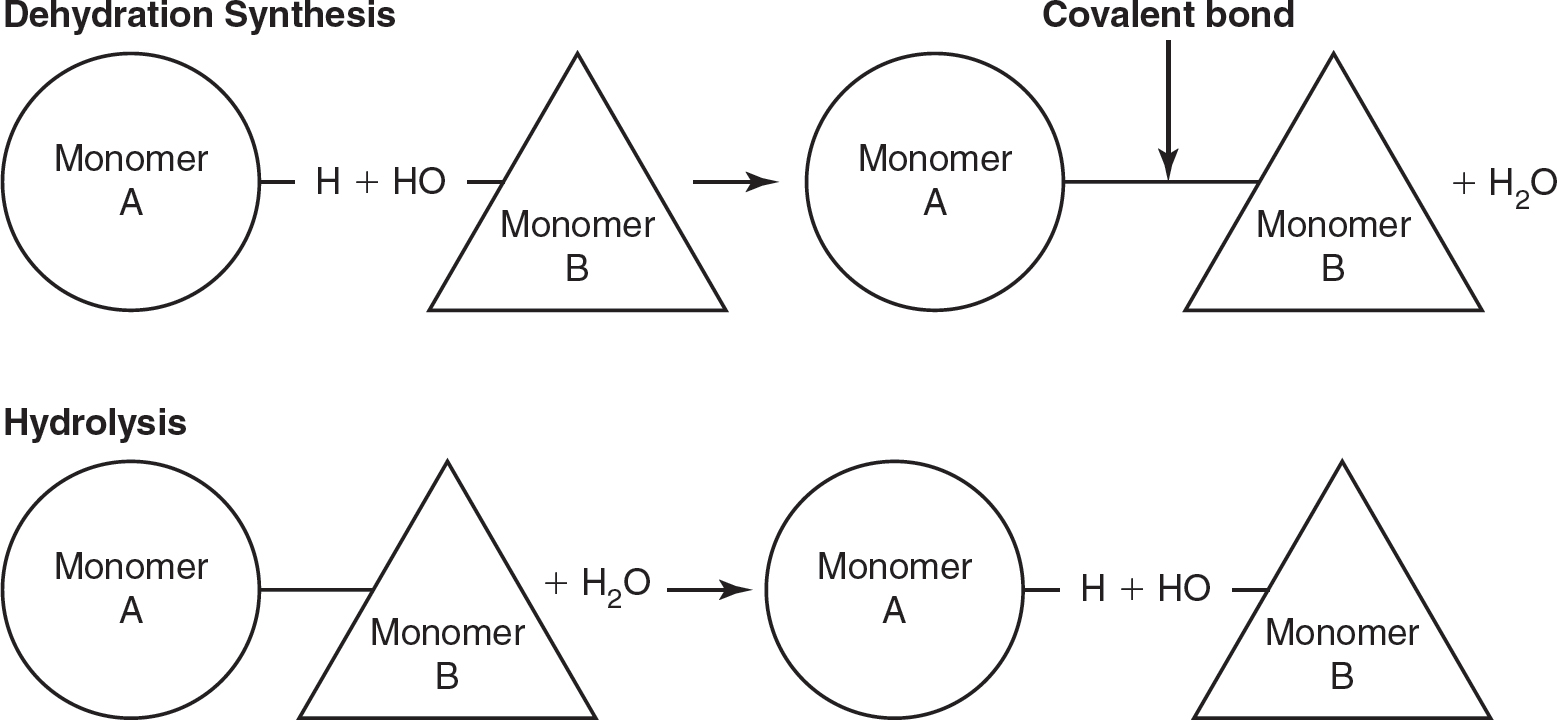
Figure 4.1 Dehydration Synthesis and Hydrolysis
Carbohydrates
Carbohydrates are polymers of sugar monomers. The types of sugars used to make the carbohydrate and how the sugars are linked determines the structure and function of the carbohydrate. The sugars may be joined in linear structures or in branched chains. Carbohydrates can be used to store energy (such as in starch or glycogen) and can also have structural functions (such as in cellulose). The type of linkages between the sugars in carbohydrates that store energy is different from the type of linkages found in carbohydrates that have a structural function. (See Figure 4.2.)
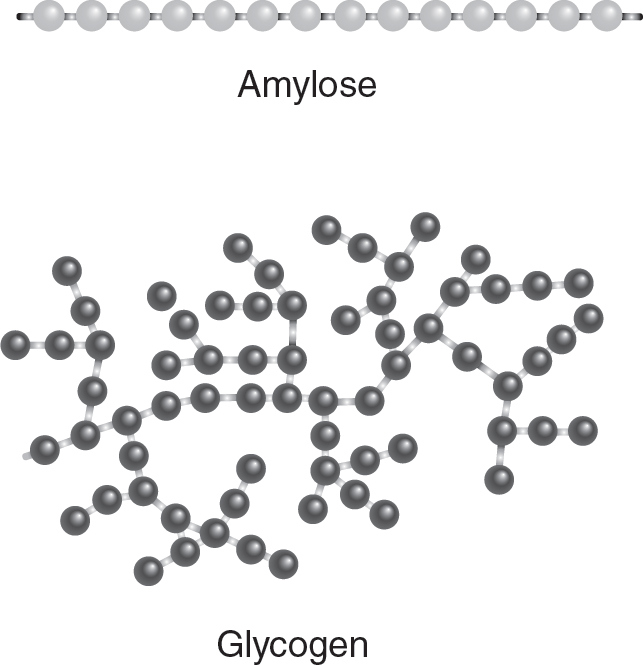
Figure 4.2 Carbohydrates
Lipids
Lipids are nonpolar macromolecules that function in energy storage, cell membranes, and insulation. (See Figure 4.3.) One of the building blocks of lipids is fatty acids. Fatty acids with the maximum number of C—H single bonds are called saturated, are solid at room temperature, and usually originate in animals. Fatty acids with at least one C=C double bond are called unsaturated, are liquid at room temperature, and usually originate in plants. How a lipid functions in a cell is dependent on the lipid’s saturation level.
Phospholipids are extremely important in cell membranes. They are built from a glycerol molecule, two fatty acids, and a phosphate group. Because the fatty acids are nonpolar and the phosphate is polar, phospholipids are amphipathic, meaning they have both hydrophobic and hydrophilic regions.
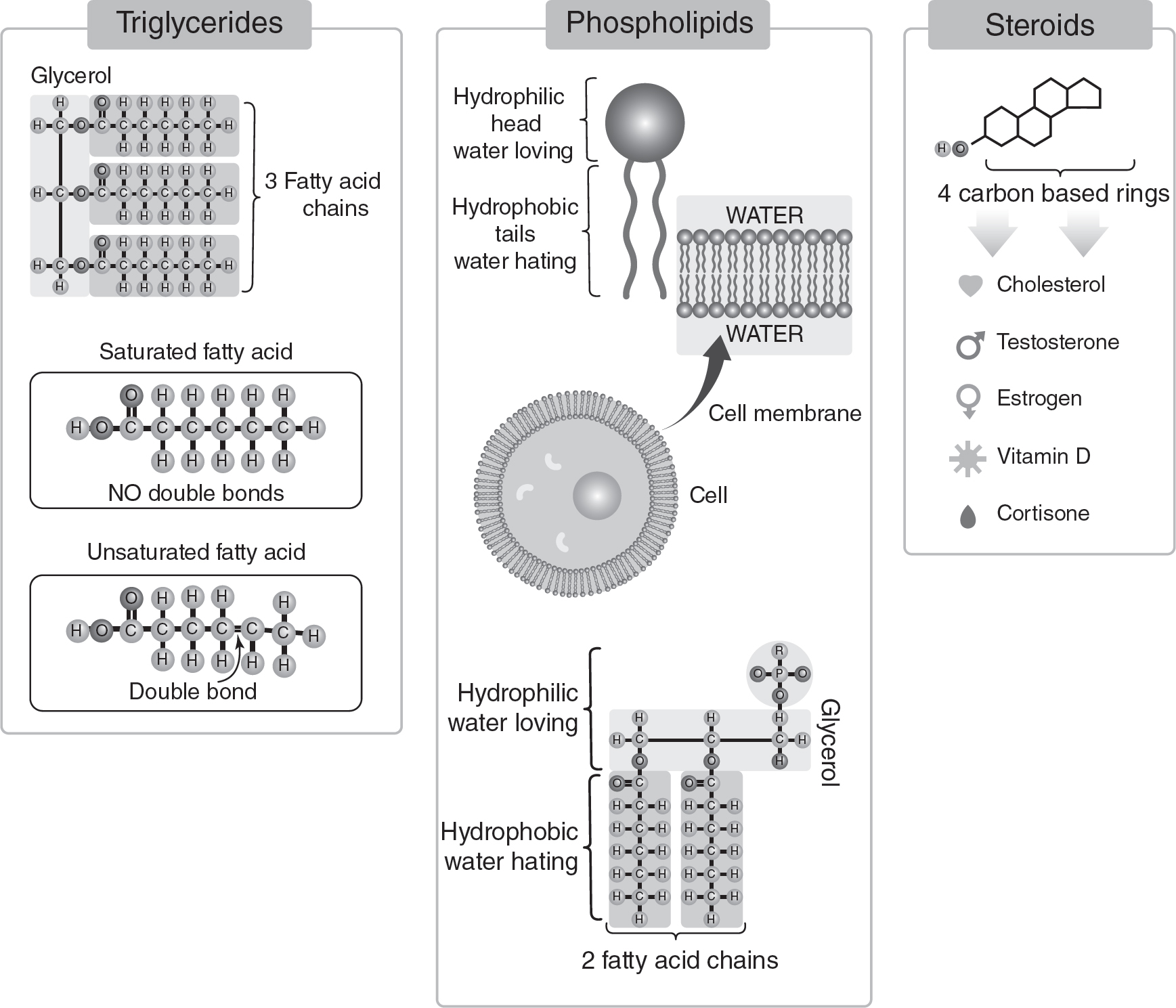
Figure 4.3 Lipids
Another class of lipids are steroids. Steroids are relatively flat, nonpolar molecules. Many steroids are formed by modifying cholesterol molecules. Examples of steroids include estradiol, testosterone, and cortisol.
Nucleic Acids
Nucleic acids are polymers of nucleotides and function as the carriers of genetic information. Nucleotides will be discussed in more detail later in this chapter.
Proteins
Proteins are polymers of amino acids. Amino acids have an amino group, a carboxylic acid group, a hydrogen atom, and a side chain (R-group) attached to a central carbon, as shown in Figure 4.4. The R-group is unique for each amino acid; it determines the amino acid’s identity and whether the amino acid will be nonpolar, polar, acidic, or basic. Proteins function in enzyme catalysis, maintaining cell structures, cell signaling, cell recognition, and more.
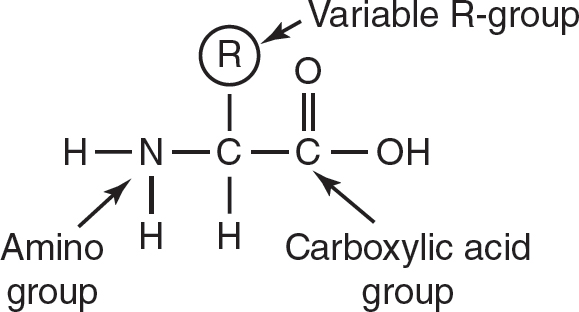
Figure 4.4 Structure of an Amino Acid
Protein Structure
There are four levels of protein structure, as shown in Figure 4.5.
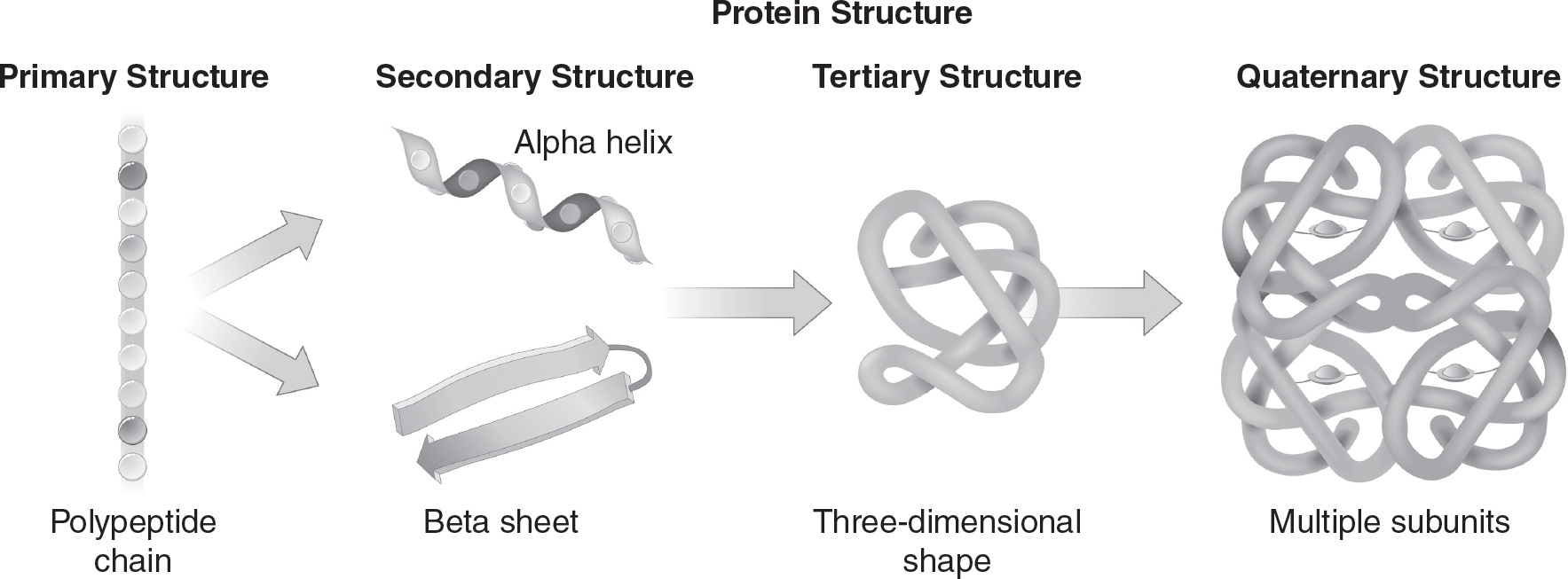
Figure 4.5 Levels of Protein Structure
Primary Structure
Amino acids are joined by peptide bonds, as shown in Figure 4.6. The resulting polypeptide chains have directionality, with an amino (NH2) terminus and a carboxyl (COOH) terminus. The order of the amino acids in the polypeptide chain determines the primary structure of the protein.
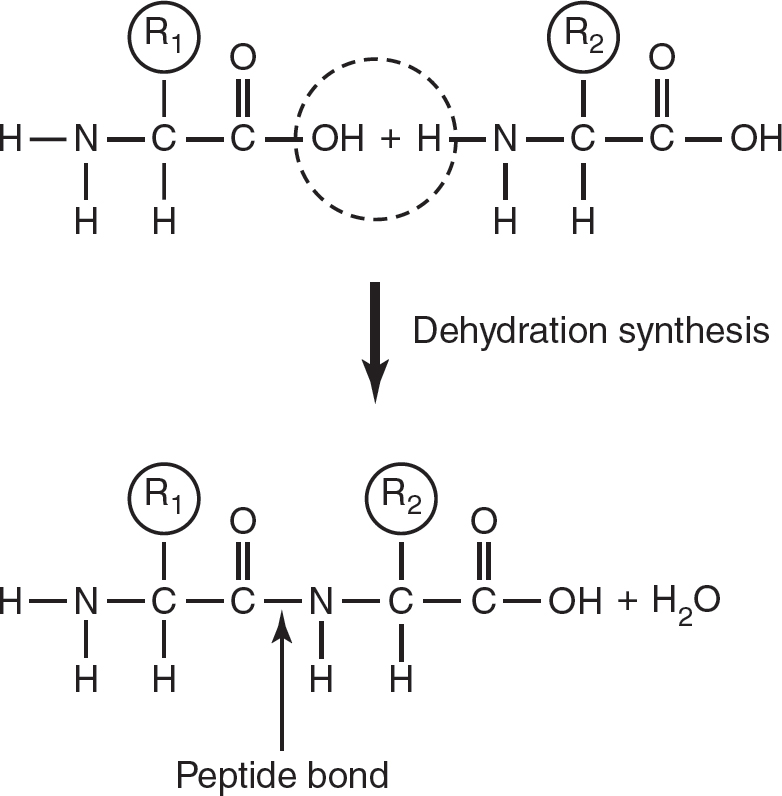
Figure 4.6 Peptide Bond
A change in the primary structure of a protein may have severe effects on the function of the protein, as seen in sickle cell disease. Just one amino acid is changed out of over 140 amino acids in sickle cell hemoglobin.
Secondary Structure
Once the primary structure is formed, hydrogen bonds may form between adjacent amino acids in the polypeptide chain. This drives the formation of the secondary structure of the protein. These secondary structures include alpha helixes and beta-pleated sheets.
Tertiary Structure
Tertiary structure is the three-dimensional folded shape of the protein, often determined by the hydrophobic/hydrophilic interactions between R-groups in the polypeptide. The most stable tertiary structures will have hydrophilic R-groups on the surface of the protein (in contact with the watery environment of the cell’s cytosol), while the amino acids with hydrophobic R-groups will be found in the interior of the protein (away from the watery cytosol). Tertiary structures may also include disulfide bridges between sulfur atoms. Special proteins called chaperonins often help fold a polypeptide into its three-dimensional structure.
Quaternary Structure
Some proteins consist of multiple polypeptide chains (subunits), which are joined together to form the complete protein and function as a unit. For example, hemoglobin has four subunits in its quaternary structure, and collagen has three subunits in its quaternary structure.
Nucleic Acids
Nucleic acids (DNA and RNA) are polymers of nucleotides, as shown in Figure 4.7. The genetic information is stored and communicated through the order of these nucleotides. Nucleotides consist of a five-carbon sugar (deoxyribose or ribose), a nitrogenous base (adenine, thymine, cytosine, guanine, or uracil), and a phosphate group. Nucleotides have directionality in that the phosphate group is always attached to the 5′ carbon in the sugar, and the 3′ carbon always has a hydroxyl group to which new nucleotides may be added.
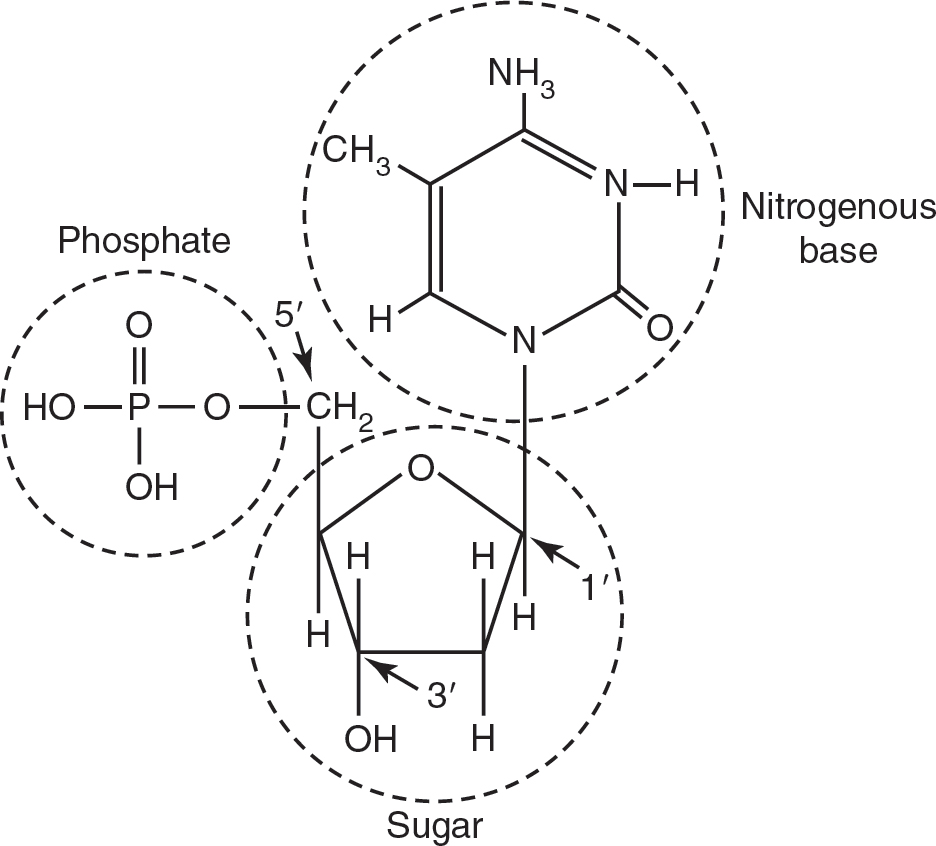
Figure 4.7 Nucleotides
While DNA and RNA are both made of nucleotides, their structures differ. Unit 6 of this book will review how these structural differences relate to their different functions. Table 4.1 summarizes some quick initial facts to know about DNA and RNA.
Table 4.1 DNA vs. RNA
|
DNA |
RNA |
|
Five-Carbon Sugar |
Deoxyribose |
Ribose |
Nitrogenous Bases |
Adenine, thymine, cytosine, and guanine |
Adenine, uracil, cytosine, and guanine |
Strands |
Double-stranded helix |
Usually single-stranded but can form three-dimensional structures when folded |
Function |
Holds genetic information |
Transcribes and regulates the expression of genetic information |
Location |
Usually found in the nucleus |
Found in both the nucleus and the cytoplasm |
The two strands in the double helix of DNA are antiparallel; the 5′ phosphate group of one of the strands in the double helix is at the opposite end from the 5′ phosphate group of the other strand, as shown in Figure 4.8.
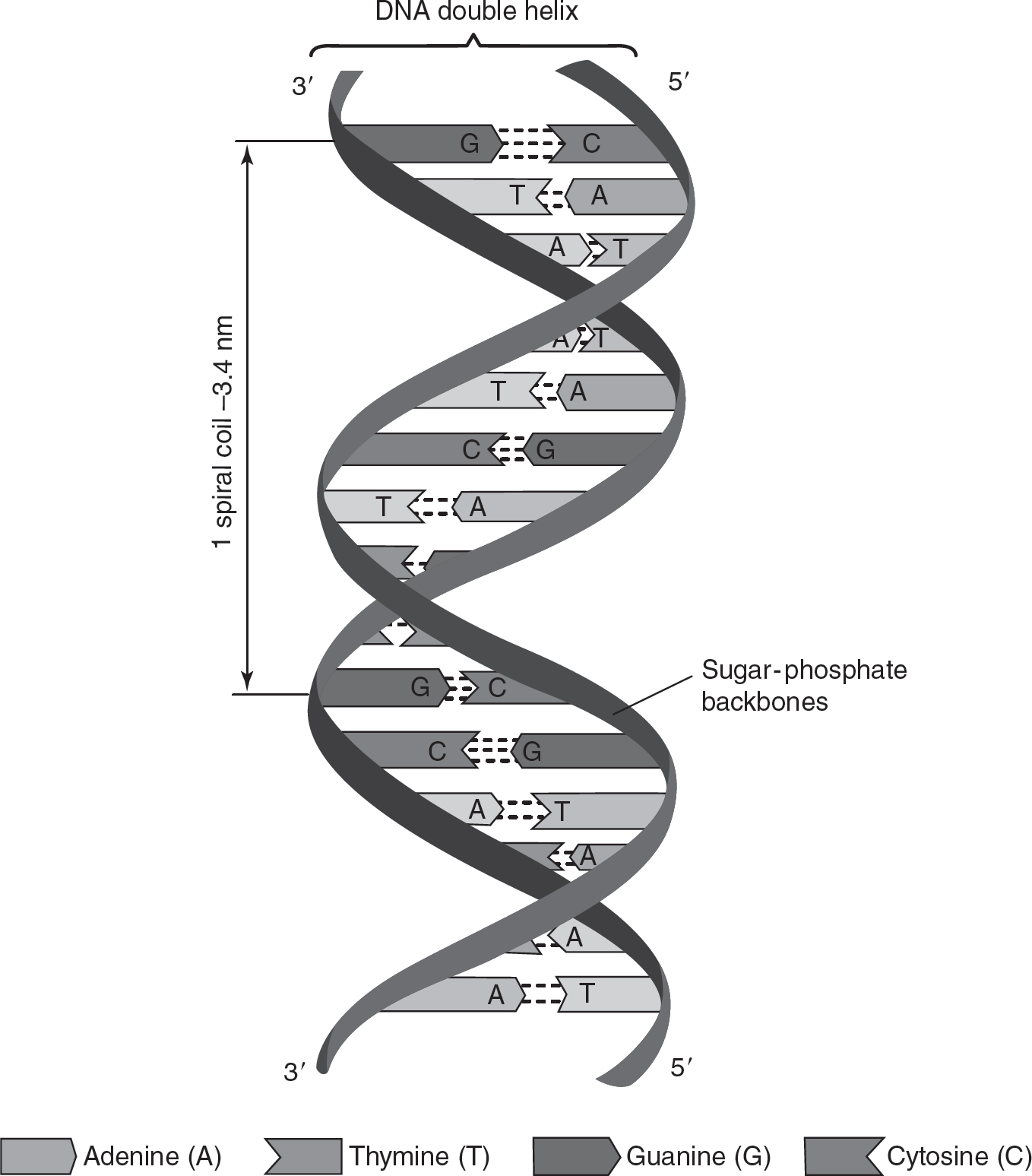
Figure 4.8 Antiparallel Structure of DNA
Thymine, uracil, and cytosine are all in a class of macromolecules called pyrimidines. Adenine and guanine are purines. When DNA strands form, a purine complements a pyrimidine and forms a series of hydrogen bonds, making the “rungs” of the ladder in the double helix. Thymine and adenine form two hydrogen bonds with one another, whereas guanine and cytosine form three hydrogen bonds with each other. Chapter 15 will discuss the profound implications this has for how DNA is replicated.
Practice Questions
Multiple-Choice
1.COVID-19 is a single-stranded RNA virus. Which molecules would most likely be found in a single-stranded RNA virus, such as COVID-19?
(A)adenine, cytosine, deoxyribose, guanine, thymine
(B)adenine, cytosine, deoxyribose, guanine, uracil
(C)adenine, cytosine, ribose, guanine, thymine
(D)adenine, cytosine, ribose, guanine, uracil
2.A scientist conducted an experiment to find out what type of macromolecule a virus injects into a cell. Using radiolabeled atoms, the scientist found that phosphorus from the virus entered the cell but sulfur did not. Which of the following molecules would most likely be injected from this virus into the cell?
(A)carbohydrate
(B)nucleic acid
(C)protein
(D)steroid
3.Which of the following best describes the formation of the primary structure of a protein?
(A)A dehydration reaction forms an ionic bond between the carboxyl group of one amino acid and the amino group of another amino acid.
(B)A dehydration reaction forms a covalent bond between the carboxyl group of one amino acid and the amino group of another amino acid.
(C)A hydrolysis reaction forms an ionic bond between the carboxyl group of one amino acid and the amino group of another amino acid.
(D)A hydrolysis reaction forms a covalent bond between the carboxyl group of one amino acid and the amino group of another amino acid.
4.Which of the following best describes the differences between saturated and unsaturated lipids?
(A)Saturated lipids have at least one C=C double bond and tend to be solid at room temperature. Unsaturated lipids have no double bonds and tend to be liquid at room temperature.
(B)Saturated lipids have at least one C=C double bond, which makes them amphipathic. Unsaturated lipids have no double bonds and are hydrophilic.
(C)Saturated lipids have no C=C double bonds and tend to be solid at room temperature. Unsaturated lipids have at least one C=C double bond and tend to be liquid at room temperature.
(D)Saturated and unsaturated lipids both have C=C double bonds. Saturated lipids are hydrophobic, and unsaturated lipids are hydrophilic.
5.The molecular formula for glucose is C6H12O6. The molecule maltose is formed by a dehydration reaction that links two glucose molecules together. What is the molecular formula for maltose?
(A)C2H4O2
(B)C6H10O5
(C)C12H22O11
(D)C12H24O12
6.In an aqueous environment like the cytosol, the most stable tertiary protein structures would have hydrophilic amino acids in which part of the protein’s structure?
(A)in the interior of the protein, interacting with water in the cytosol
(B)in the interior of the protein, avoiding water in the cytosol
(C)on the surface of the protein, interacting with water in the cytosol
(D)on the surface of the protein, avoiding water in the cytosol
7.Which level of protein structure is formed by peptide bonds between amino acids?
(A)primary
(B)secondary
(C)tertiary
(D)quaternary
8.Which of the following drives the formation of a protein’s secondary structure?
(A)Hydrophobic interactions form between R-groups of amino acids.
(B)Hydrogen bonds form between amino acids in a polypeptide chain.
(C)Disulfide bridges form between amino acids in a polypeptide chain.
(D)Multiple subunits/domains of a protein are connected by covalent bonds.
9.Which of the following is common to both DNA and RNA?
(A)the nitrogenous bases: adenine, cytosine, guanine, and thymine
(B)a double-stranded antiparallel helix
(C)a phosphate group attached to the 5′ carbon
(D)the five-carbon sugar ribose
10.Which of the following correctly describes DNA but not RNA?
(A)It contains adenine, cytosine, guanine, and uracil.
(B)A hydroxyl group is attached to the 5′ carbon.
(C)Nucleotides are linked by phosphodiester bonds.
(D)It contains the five-carbon sugar deoxyribose.
Short Free-Response
11.A student conducts an experiment to compare the melting points of an unsaturated fatty acid and a saturated fatty acid. The data are shown in the table.
|
Fatty Acid |
Melting Point (°C) |
Oleic acid (unsaturated) |
16.3 |
Stearic acid (saturated) |
69.3 |
(a)Describe the structural differences between an unsaturated fatty acid and a saturated fatty acid.
(b)Identify the independent variable and the dependent variable in this experiment.
(c)The membrane surrounding cell A contains a much higher percentage of unsaturated fatty acids than the membrane surrounding cell B. Predict which cell would have a more flexible membrane.
(d)Justify your prediction from part (c).
12.The melting temperature (Tm) is defined as the temperature at which 50% of double-stranded DNA is separated into single-stranded DNA. The greater the guanine-cytosine (poly-GC) of the DNA, the higher the Tm compared to DNA with more adenine-thymine (poly-AT) content. The following graph shows the Tm of a poly-AT DNA strand, a poly-GC DNA strand, and DNA from two different organisms (A and B).
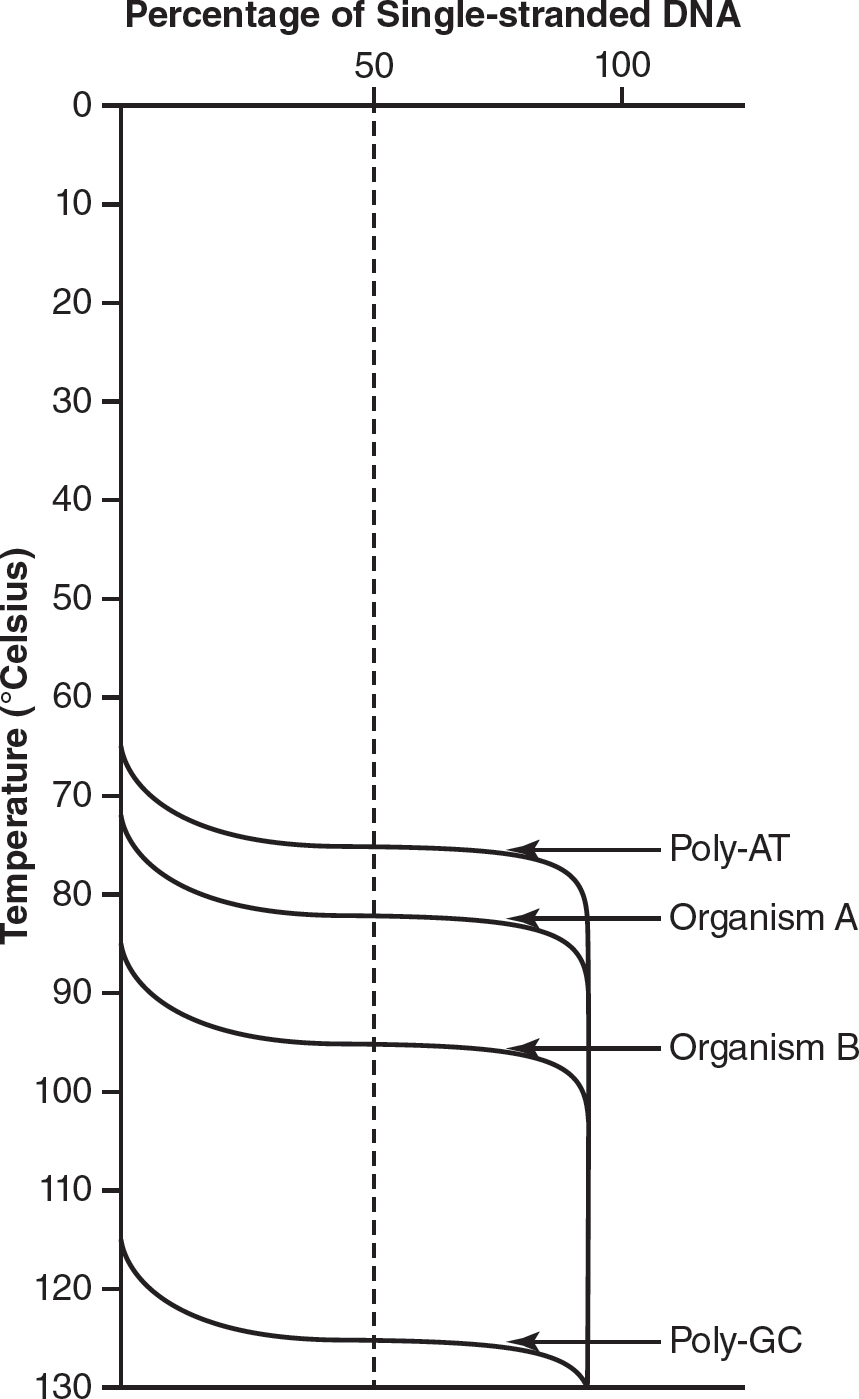
(a)Describe how the Tm of DNA from organism A compares to the Tm of DNA from organism B.
(b)Describe the differences between the Tm of poly-AT and the Tm of poly-GC DNA.
(c)DNA sequencing finds that DNA from organism A has a GC content of 39% and that DNA from organism B has a GC content of 48%. A student claims that DNA from organism C (with a GC content of 55%) would have a Tm greater than 85° Celsius. Using the data from the figure, evaluate the student’s claim.
(d)Explain how these experimental results relate to the structure of DNA.
Long Free-Response
13.Genomes with a higher GC content are more likely to resist denaturation. A plant biologist hypothesized that plants with a higher GC content in their genomes are more likely to be found in colder climates. Four species of plants were examined for the percentage of GC content in their genomes. Brachypodium pinnatum and Dioscorea caucasica are native to areas with cold winters. Juncus inflexus and Carex acutiformis are native to areas with warmer winters. DNA was examined from 50 plants of each species. Results are shown in the table.
|
Plant Species |
Mean Percent of GC Content |
Standard Error of the Mean |
Brachypodium pinnatum |
46.0 |
1.9 |
Dioscorea caucasica |
42.5 |
2.0 |
Juncus inflexus |
33.7 |
2.0 |
Carex acutiformis |
35.6 |
0.8 |
(a)Using what you know about DNA structure, explain why genomes with a higher GC content might be more stable than genomes with a lower GC content.
(b)On the axes provided, construct an appropriately labeled graph of the mean percent of GC content in each species’s genome. Include 95% confidence intervals.
(c)Do the data support the claim that plants in colder climates have a higher GC content in their genomes? Use the data provided to support your answer.
(d)A plant species has a genome with a GC content of 43.4%. Predict whether that plant is more likely to be native to an area with cold winters or an area with warmer winters. Justify your prediction.
Answer Explanations
Multiple-Choice
1.(D)RNA contains ribose and uracil. Choice (A) is incorrect because deoxyribose and thymine are found in DNA, not RNA. Deoxyribose is found in DNA, not RNA, so choice (B) is incorrect. Choice (C) is incorrect because thymine is not found in RNA; it is found in DNA.
2.(B)Nucleic acids contain phosphorus but not sulfur. So if phosphorus entered the cell but sulfur did not, a nucleic acid was most likely injected. Choice (A) is incorrect because carbohydrates typically do not contain phosphorus. Proteins typically contain sulfur but not phosphorus, so choice (C) is incorrect. Choice (D) is incorrect because steroids are a type of lipid that do not contain phosphorus.
3.(B)The primary structure of a protein is formed by linking amino acids, and dehydration reactions link amino acids in covalent bonds between the carboxyl group of one amino acid and the amino group of another amino acid. Choice (A) is incorrect because dehydration reactions do not form ionic bonds. Choices (C) and (D) are incorrect because hydrolysis reactions break, not form, bonds between monomers.
4.(C)Saturated lipids have carbon atoms linked through single bonds and form straight chains; this makes saturated lipids easier to pack tightly and more likely to be solid at room temperature. Choice (A) is incorrect because saturated lipids do not have C=C double bonds. Choice (B) is incorrect because saturated lipids are not amphipathic (phospholipids are amphipathic). Choice (D) is incorrect because while unsaturated lipids do have C=C double bonds, saturated lipids do not. Also, both saturated and unsaturated lipids are hydrophobic.
5.(C)A dehydration reaction removes a water molecule. Two glucose molecules would contain 12 carbon atoms, 24 hydrogen atoms, and 12 oxygen atoms, but the dehydration reaction (linking the two glucose molecules to form maltose) would remove two hydrogen atoms and one oxygen atom to form the water molecule removed in a dehydration reaction. This leaves 12 carbon atoms but only 22 hydrogen atoms and 11 oxygen atoms. Choice (A) is incorrect because it has fewer atoms than a single glucose molecule, so it could not be maltose (which is made from two glucose molecules). Choice (B) is incorrect because it is what would remain if a water molecule was removed from a single glucose molecule, not from two glucose molecules. Choice (D) is incorrect because it shows the number of atoms present in two glucose molecules without taking into account the atoms lost from the water molecule that was removed in the dehydration synthesis reaction.
6.(C)Tertiary protein structures in the cell are most stable when their hydrophilic amino acids are on the surface of the protein, in contact with the watery cytosol of the cell. Choices (A) and (B) are incorrect because hydrophobic amino acids are more likely to be found in the interior of a protein, away from water in the cytosol. Choice (D) is incorrect because hydrophilic amino acids would not avoid water; they would be more stable when interacting with water.
7.(A)The primary structure of a protein is the sequence of amino acids held together by peptide bonds. Choice (B) is incorrect because secondary structure is formed by the hydrogen bonds between amino acids in a polypeptide chain. Tertiary structure is the globular shape formed by a polypeptide chain, so choice (C) is incorrect. Choice (D) is incorrect because quaternary structure is formed when multiple subunits come together to form the functional protein.
8.(B)Secondary structure is driven by the formation of hydrogen bonds between the carboxyl groups and amino groups in a polypeptide chain. Choice (A) is incorrect because hydrophobic interactions between R-groups influence tertiary and quaternary structures but not secondary structures. Choice (C) is incorrect because disulfide bridges are formed in tertiary and quaternary structures, not secondary structures. Choice (D) is incorrect because multiple subunits/domains are only present in quaternary structures.
9.(C)Both DNA and RNA have a phosphate group attached to the 5′ carbon. Choice (A) is incorrect because RNA does not contain thymine. A double-stranded antiparallel helix is only present in DNA, not RNA, so choice (B) is incorrect. Choice (D) is incorrect because ribose is present in RNA but not in DNA.
10.(D)Deoxyribose is found in DNA; ribose is found in RNA. Choice (A) is incorrect because DNA does not contain uracil. Choice (B) is incorrect because the hydroxyl group is attached to the 3′ carbon, not the 5′ carbon. Phosphodiester bonds are found in both DNA and RNA, so choice (C) is incorrect.
Short Free-Response
11.(a)Saturated fatty acids have a chain of carbon atoms connected by single bonds. Unsaturated fatty acids have carbon chains that contain at least one double bond between the carbon atoms.
(b)The independent variable is the type of fatty acid. The dependent variable is the melting point in degrees Celsius.
(c)Cell A would have a more flexible cell membrane.
(d)Cell A would have a more flexible cell membrane because it contains more unsaturated fatty acids. Unsaturated fats contain double bonds in random locations, causing bends in the long chains. These bends in the fatty acid chains prevent unsaturated fats from forming tightly packed sheets, which leaves some spaces between the fatty acid chains. Saturated fats, on the other hand, are straight chains; they form tightly packed sheets and have less space between their fatty acid chains. Since unsaturated fats cannot pack tightly like saturated fats, this allows unsaturated fats to be more flexible and thus be liquid at room temperature.
12.(a)The Tm of DNA from organism A is lower than the Tm of DNA from organism B.
(b)The Tm of poly-GC DNA is much higher than the Tm of poly-AT DNA.
(c)DNA from organism B has a GC content of 48% and a Tm of approximately 85° Celsius. Since increased GC content leads to a higher Tm and the DNA from organism C has a GC content of 55%, it would follow the pattern of the data that organism C’s Tm would be higher than 85° Celsius.
(d)In DNA, GC pairs form three hydrogen bonds while AT pairs form only two hydrogen bonds. More hydrogen bonds would require more energy to separate, so it makes sense that DNA with a higher GC content would have a higher Tm.
Long Free-Response
13.(a)The two strands of a DNA double helix are held together by hydrogen bonds between the base pairs. AT pairs form two hydrogen bonds, and the GC pairs form three hydrogen bonds. Since GC pairs form more hydrogen bonds, that may make DNA with a higher GC content more stable across a wider range of temperatures than DNA with a lower GC content.
(b)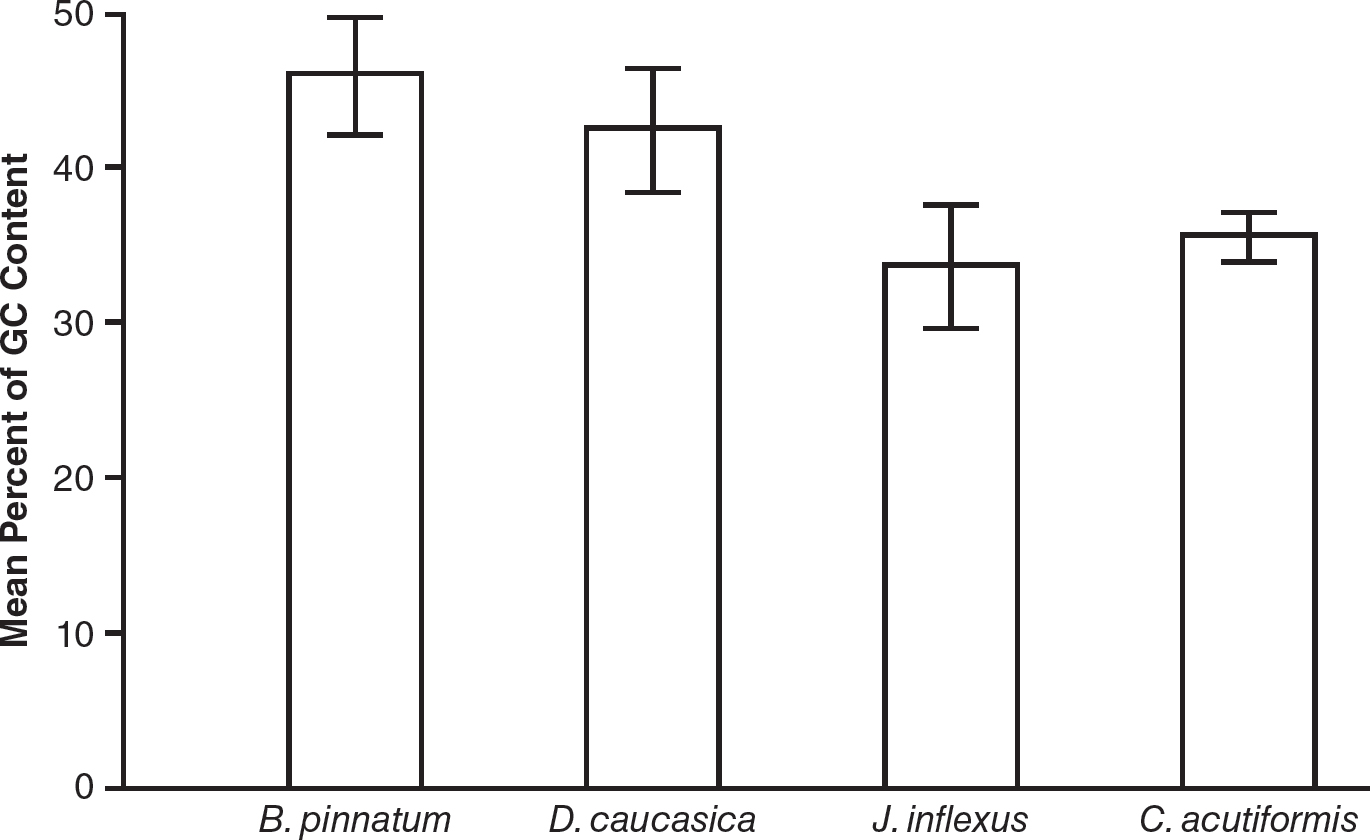
(c)Yes, the claim that plants in colder climates have a higher GC content in their genomes is supported by the data. B. pinnatum and D. caucasica are native to colder climates. Both also have statistically significantly higher GC content in their genomes as compared to the species found in warmer winter locations (J. inflexus and C. acutiformis), as shown in the graph for part (b).
(d)The plant with a GC content of 43.4% is more likely be native to an area with cold winters since its GC content is closer to the GC contents found in B. pinnatum and D. caucasica.