Biology Premium, 2024: 5 Practice Tests + Comprehensive Review + Online Practice - Wuerth M. 2023
UNIT 3 Cellular Energetics
9 Cellular Respiration
Learning Objectives
In this chapter, you will learn:
➜Glycolysis
➜Oxidation of Pyruvate
➜Krebs Cycle (Citric Acid Cycle)
➜Oxidative Phosphorylation
➜Fermentation
Overview
Chapter 8 reviewed how photosynthetic organisms harness light energy and store it in the chemical bonds of organic molecules. This chapter will review how living organisms release the energy stored in the chemical bonds of organic molecules to drive the processes necessary for life.
The overall equation for cellular respiration is:
![]()
Cellular respiration includes the following cellular processes: glycolysis, the oxidation of pyruvate, the Krebs cycle (also known as the citric acid cycle), and oxidative phosphorylation, all of which will be discussed in more detail later in this chapter.
The presence or absence of oxygen determines which cellular processes a living organism can use to obtain energy from food. Anaerobic organisms, which do not have access to or do not require oxygen, can perform glycolysis and fermentation. Aerobic organisms that do not have access to oxygen can also perform glycolysis and fermentation. In addition, in the presence of oxygen, aerobic organisms can also perform the oxidation of pyruvate, the Krebs cycle, and oxidative phosphorylation. Table 9.1 summarizes the differences between anaerobic organisms and aerobic organisms in the presence or absence of oxygen.
Table 9.1 Cellular Processes Performed by Anaerobic and Aerobic Organisms
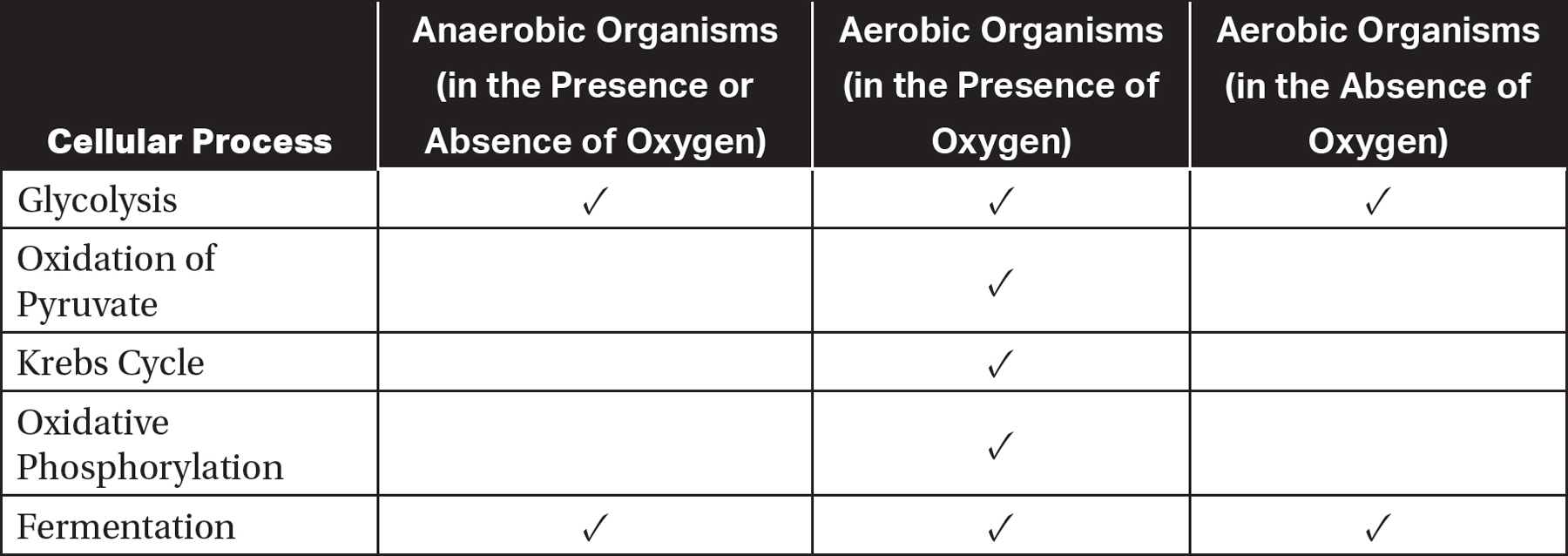
Aerobic organisms can perform more metabolic processes than anaerobic organisms can perform. This allows aerobic organisms to extract much more energy from organic compounds than anaerobic organisms can.
First is a review of the major cellular processes of cellular respiration, which is then followed by a review of fermentation.
As you review each cellular process, it is not necessary to memorize every step and enzyme involved! Focus on the flow of electrons and energy through the process. For each process (glycolysis, oxidation of pyruvate, the Krebs cycle, oxidative phosphorylation, and fermentation), know where it happens and what the inputs and outputs are for each.
Glycolysis
Glycolysis occurs in the cytosol of the cell. Since all living organisms have cytosol, all living organisms can perform glycolysis. Glycolysis was probably one of the first metabolic processes to evolve. This theory is supported by the fact that enzymes, which catalyze the steps of glycolysis, are highly conserved and are found in all living organisms.
The six-carbon molecule glucose enters glycolysis, along with two molecules of the electron carrier NAD+. During glycolysis, the glucose molecule is oxidized (loses hydrogen atoms and their electrons), and each NAD+ is reduced (gains a hydrogen atom and its electrons) to NADH. Remember that whenever one molecule is oxidized, another molecule must be reduced. During cellular respiration, the molecules that contain carbon are oxidized and the electron carriers NAD+ and FAD+ are reduced.
TIP
Remember to follow the carbons: While you do not need to know the structures of glucose and pyruvate, know how many carbons are in each molecule.
Two molecules of ATP are required in the early steps of glycolysis. However, four molecules of ATP are produced by glycolysis, resulting in a net gain of two ATP molecules.
At the end of glycolysis, the six-carbon glucose molecule is cleaved into two three-carbon pyruvate molecules.
Glycolysis is a multistep process that involves many enzyme-catalyzed steps and intermediates. When studying for the AP Biology exam, focus on where each process in cellular respiration occurs and what the inputs and outputs are for each process. Figure 9.1 and Table 9.2 summarize the location, inputs, and outputs of glycolysis.
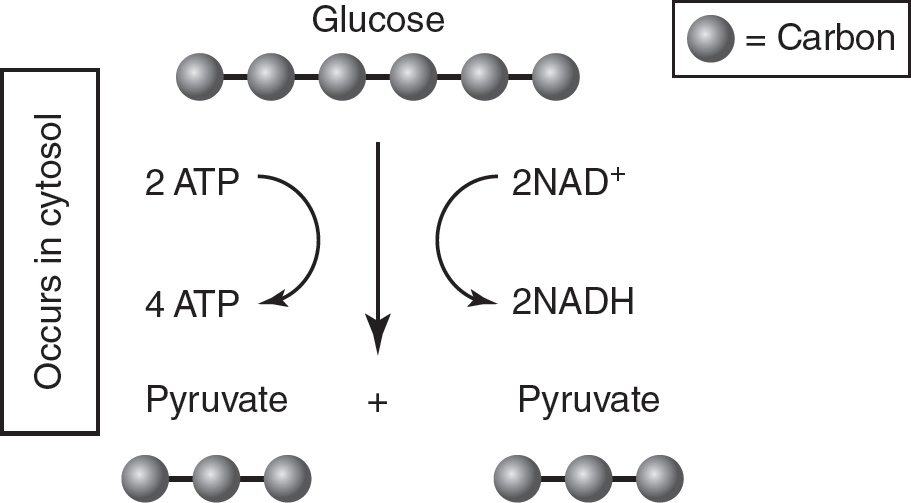
Figure 9.1 Glycolysis
Table 9.2 Glycolysis
|
Location |
Inputs |
Outputs |
Cytosol |
Glucose (6C) |
2 Pyruvate (3C) |
2 NAD+ |
2 NADH |
|
2 ATP |
4 ATP |
Oxidation of Pyruvate
The next step in cellular respiration occurs in the mitochondria. The three-carbon pyruvate molecule must be modified in order to enter the mitochondria. Pyruvate is oxidized (loses a hydrogen atom and its electrons), and the electron carrier NAD+ is reduced (gains a hydrogen atom and its electrons) and becomes NADH. As this happens, one of the carbons in the pyruvate molecule is released as carbon dioxide, leaving behind a two-carbon acetyl group. Coenzyme A attaches to this two-carbon acetyl group. Coenzyme A will deliver the acetyl group to the Krebs cycle.
Each molecule of glucose that enters glycolysis will generate two molecules of pyruvate, so the oxidation of pyruvate will occur twice for each molecule of glucose that entered glycolysis. Figure 9.2 and Table 9.3 summarize the location, inputs, and outputs of the oxidation of pyruvate.
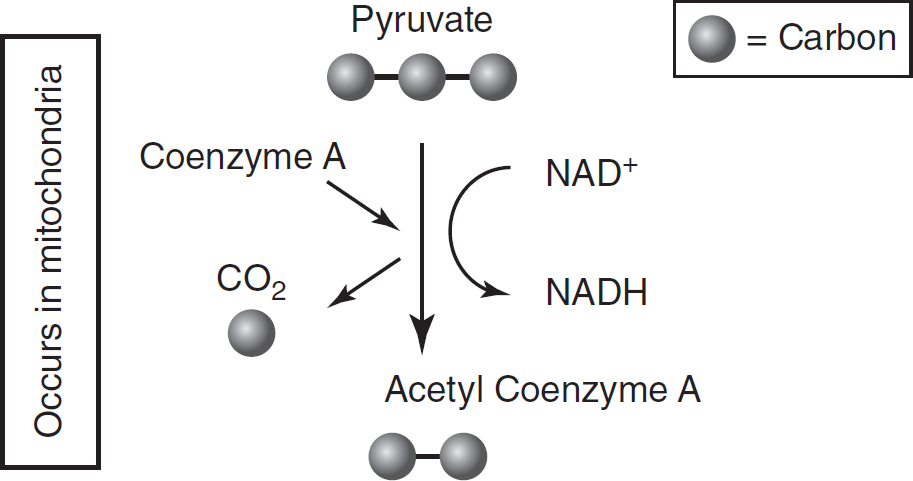
Figure 9.2 Oxidation of Pyruvate
Table 9.3 Oxidation of Pyruvate
|
Location |
Inputs |
Outputs |
Mitochondria |
Pyruvate (3C) |
Acetyl group (2C) |
NAD+ |
Carbon dioxide (1C) |
|
NADH |
Krebs Cycle (Citric Acid Cycle)
The Krebs cycle (also known as the citric acid cycle) occurs in the matrix (the liquid center) of the mitochondria. Coenzyme A brings the two-carbon acetyl group to the Krebs cycle, where it is initially attached to a four-carbon intermediate, forming a six-carbon molecule. This six-carbon molecule goes through a series of enzyme-catalyzed reactions, during which two more carbon dioxide molecules are released and the four-carbon intermediate is regenerated. At the completion of the cycle, all of the carbon that was originally in the glucose molecule at the start of glycolysis has been released as carbon dioxide. (See Figure 9.3 and Table 9.4.)
During one turn of the Krebs cycle, four electron carriers are reduced. Three molecules of NAD+ are reduced to NADH, and one molecule of FAD+ is reduced to FADH2. In addition, one molecule of ATP is produced through substrate-level phosphorylation (the direct addition of a phosphate group to ADP without the use of an electron transport chain or chemiosmosis).
There are two methods of ATP production you need to understand for the AP Biology exam: substrate-level phosphorylation and oxidative phosphorylation. Substrate-level phosphorylation is a simpler process, but oxidative phosphorylation allows for the production of greater amounts of ATP.
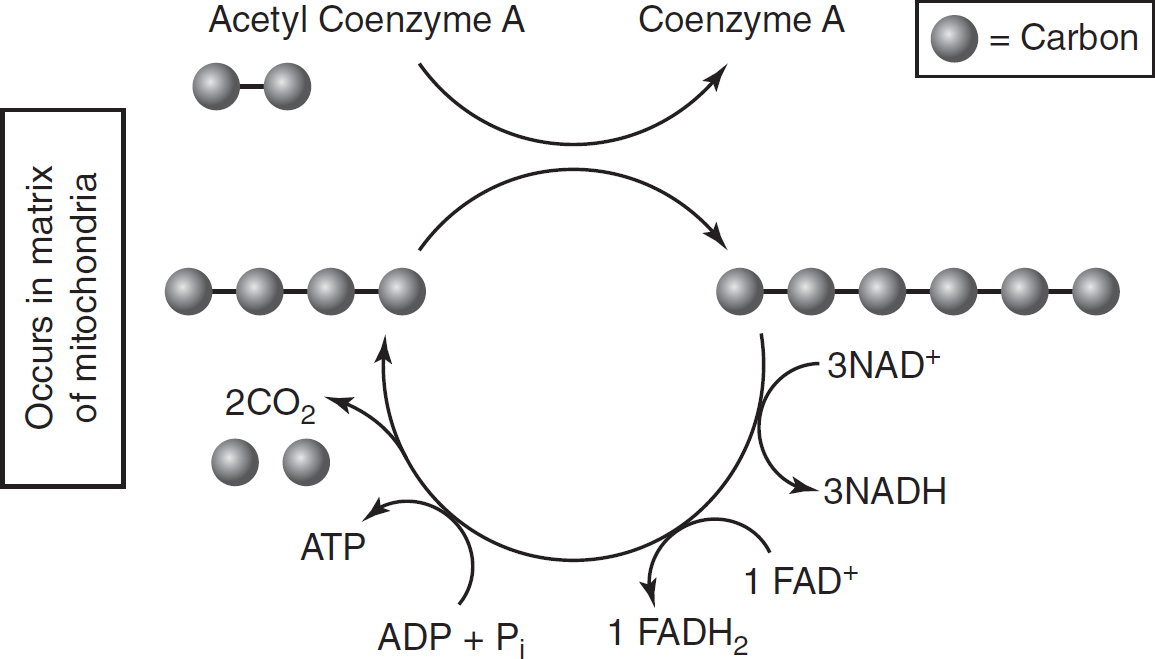
Figure 9.3 Krebs Cycle
Table 9.4 Krebs Cycle
|
Location |
Inputs |
Outputs |
|
Matrix of the mitochondria |
Acetyl group (2C) |
2 carbon dioxides (1C each) |
3 NAD+ |
3 NADH |
|
1 FAD+ |
1 FADH2 |
|
1 ADP + Pi |
1 ATP |
TIP
Glycolysis takes in a six-carbon glucose and produces two three-carbon pyruvates. For this reason, while glycolysis occurs once per glucose molecule, both the oxidation of pyruvate and the Krebs cycle occur twice per glucose molecule.
Before moving on to oxidative phosphorylation, review Table 9.5 for a recap of what has been generated for each glucose molecule that entered cellular respiration.
Table 9.5 Total Products of Glycolysis, Oxidation of Pyruvate, and the Krebs Cycle for Each Glucose Molecule that Entered Cellular Respiration
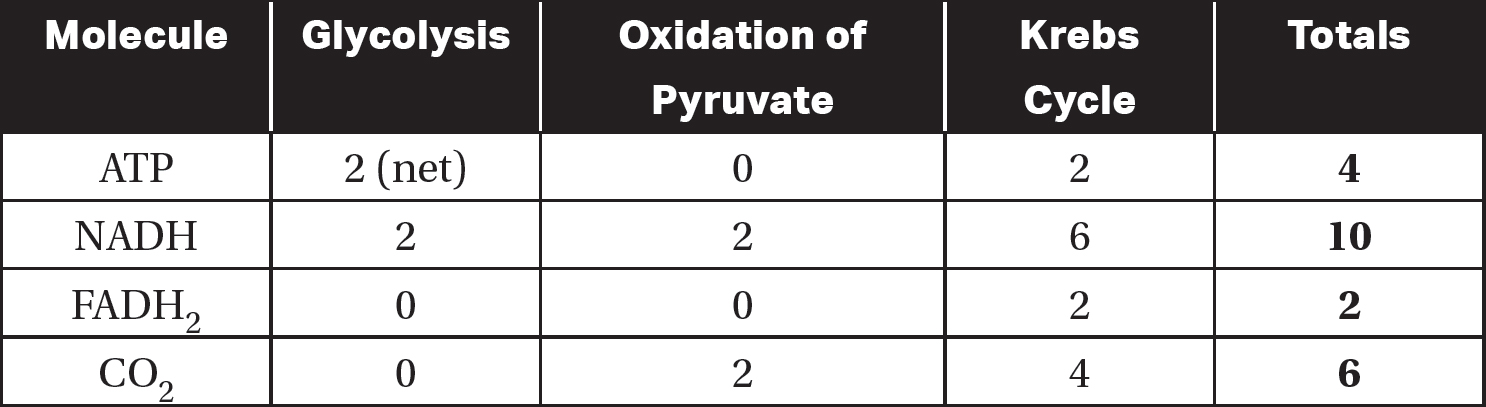
When reviewing Table 9.5, notice that all six carbons in the glucose molecule that entered cellular respiration have been released as carbon dioxide. Four molecules of ATP have been produced by substrate-level phosphorylation (two in glycolysis and two in the Krebs cycle). A total of 12 high-energy electron carriers (10 NADH and two FADH2) have been produced and will enter the next stage of cellular respiration: oxidative phosphorylation.
Oxidative Phosphorylation
Oxidative phosphorylation involves the electron transport chain (ETC) and chemiosmosis, both of which occur on the inner membrane of the mitochondria. Oxidative phosphorylation yields the vast majority of ATP in cellular respiration.
The electron carriers (NADH and FADH2) that were generated during glycolysis, the oxidation of pyruvate, and the Krebs cycle bring their electrons to the electron transport chain on the inner mitochondrial membrane. (See Figure 9.4.) As these electron carriers deliver their hydrogen atoms and electrons to the ETC, NADH and FADH2 are oxidized to NAD+ and FAD+, respectively. NAD+ and FAD+ can then be reused in the earlier processes of cellular respiration.
As the electrons travel through the electron transport chain, their potential energy decreases, and energy is released. This energy is used to pump protons (H+) out of the matrix and into the intermembrane space of the mitochondria, creating a proton gradient. The concentration of protons in the intermembrane space can be 1,000 times that of the matrix!
At the end of the electron transport chain, molecular oxygen (O2) combines with four protons (H+) and four electrons (e—) to form two water molecules. This makes oxygen the final, or terminal, electron acceptor during cellular respiration.
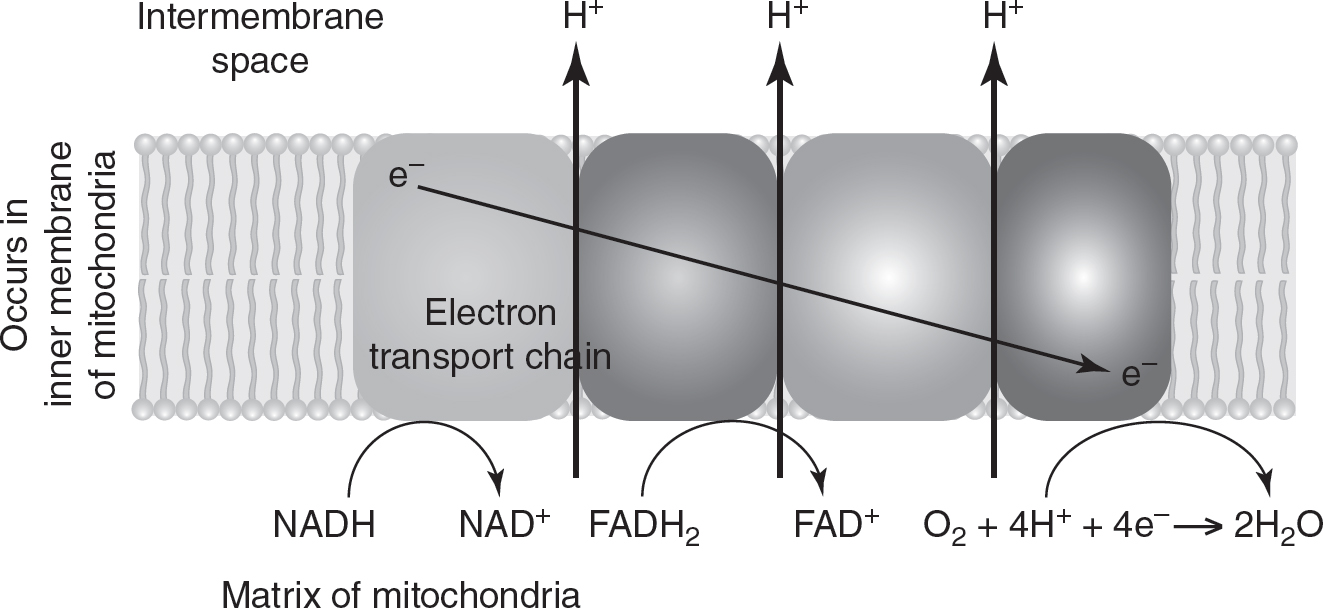
Figure 9.4 Electron Transport Chain
The proton gradient created by the electron transport chain is used to drive ATP synthesis. Using a proton gradient to drive the production of ATP is called chemiosmosis, as shown in Figure 9.5. The enzyme ATP synthase catalyzes this process. ATP synthase is located on the inner membrane of the mitochondria. Protons flow from an area of higher concentration in the intermembrane space to an area of lower concentration in the matrix through a channel in the ATP synthase enzyme. This flow of protons down their concentration gradient through ATP synthase causes a shape change in the enzyme. This change in shape allows ATP synthase to catalyze the production of ATP.
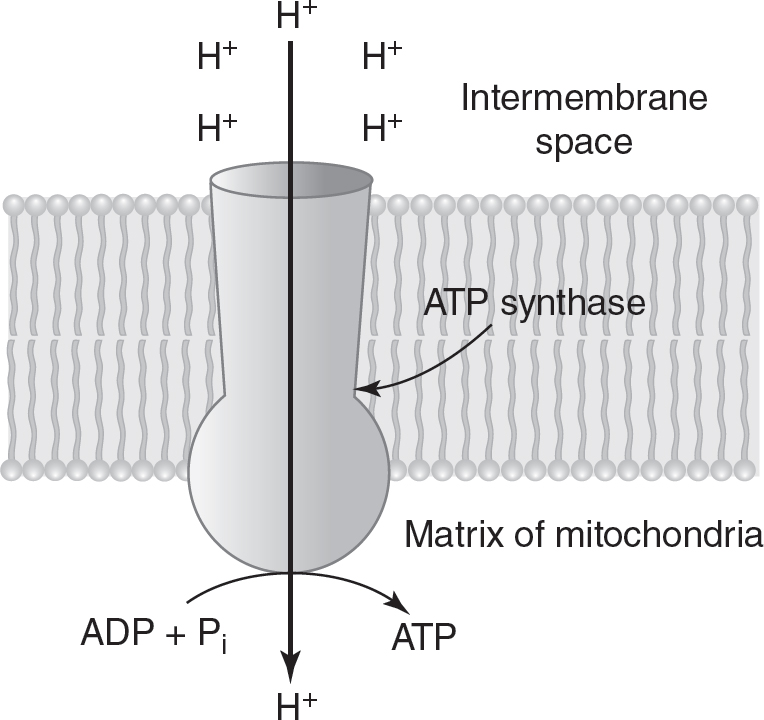
Figure 9.5 Chemiosmosis Produces ATP
Ideally, each NADH that enters the electron transport chain can generate as many as three ATPs. Each FADH2 that enters the electron transport chain can generate as many as two ATPs; this is because FADH2 has less potential energy than NADH and enters the electron transport chain at a later point than NADH. Using the totals from previous processes of cellular respiration (as presented in Table 9.5), the number of ATPs that can be generated by oxidative phosphorylation can be calculated as follows:

Therefore, oxidative phosphorylation can generate 34 ATPs. Compare that to the net gain of two ATPs from glycolysis and the yield of two ATPs from the Krebs cycle to see how much more productive oxidative phosphorylation is with regard to ATP production! However, in living organisms, metabolic processes rarely work with 100% efficiency. For example, some membranes may be “leaky,” and some protons may cross the inner membrane of the mitochondria without going through ATP synthase. So actual yields from this process may vary.
Fermentation
During oxidative phosphorylation, NADH is oxidized (at the electron transport chain) to NAD+, which can then be returned to be used in glycolysis. However, if oxygen is not present, oxidative phosphorylation cannot occur. (Recall that oxygen is the final electron acceptor in the ETC. Without oxygen present, the ETC cannot release its low-energy electrons from the final carrier, blocking the chain and shutting the entire system down.) In anaerobic conditions, cells carry out fermentation to regenerate the NAD+ needed to keep the process of glycolysis going. If a cell ran out of NAD+, it could no longer perform glycolysis. All generation of ATP would stop, resulting in the death of the cell.
Fermentation occurs only in the cytosol. The two major types of fermentation you need to know are shown in Figures 9.6 and 9.7: alcohol fermentation and lactic acid fermentation.
In alcohol fermentation, pyruvate is reduced to an alcohol (typically the two-carbon alcohol ethanol) and carbon dioxide, and NADH is oxidized to NAD+. An example of this is the fermentation that yeast undergoes as bread dough rises. As yeast ferments the sugars in the uncooked bread dough, alcohol and carbon dioxide are produced. The carbon dioxide gas causes the bread dough to “rise,” and the alcohol is evaporated by high temperatures during the baking of the bread dough.
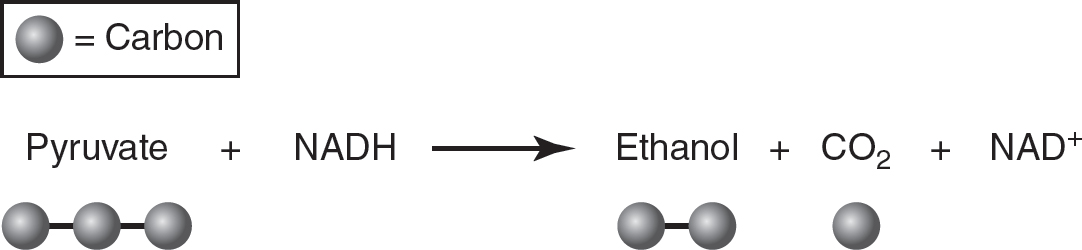
Figure 9.6 Alcohol Fermentation
In lactic acid fermentation, pyruvate is reduced to lactic acid (a three-carbon molecule), and NADH is oxidized to NAD+. No carbon dioxide is produced. Lactic acid fermentation can occur in muscle cells if they do not have enough oxygen to carry out oxidative phosphorylation.
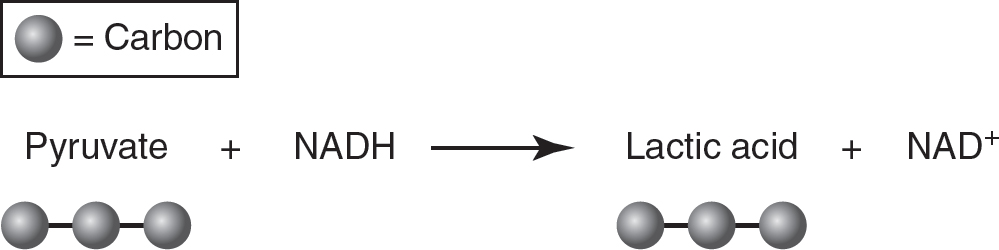
Figure 9.7 Lactic Acid Fermentation
Practice Questions
Multiple-Choice
1.What is the primary purpose of fermentation?
(A)to generate ATP
(B)to generate pyruvate
(C)to generate NAD+
(D)to generate carbon dioxide
2.Which of the following processes does NOT release carbon dioxide?
(A)glycolysis
(B)oxidation of pyruvate
(C)Krebs cycle
(D)alcohol fermentation
3.Which of the following statements about glycolysis is NOT correct?
(A)Glycolysis occurs in the cytosol.
(B)Glycolysis can be carried out by all living organisms.
(C)The enzymes of glycolysis are highly conserved.
(D)Glycolysis produces a proton gradient.
4.A mutation in mitochondrial DNA causes the creation of a pore in the mitochondrial membrane through which protons can freely pass. Which of the following processes would most likely be disrupted by this mutation?
(A)glycolysis
(B)Krebs cycle
(C)chemiosmosis
(D)fermentation
5.During oxidative phosphorylation, ____________ is ____________, and oxygen is ____________.
(A)NADH; oxidized; produced
(B)NADH; oxidized; reduced
(C)NAD+; reduced; oxidized
(D)NAD+; reduced; produced
6.Which of the following processes produce ATP?
(A)glycolysis, oxidation of pyruvate, Krebs cycle
(B)glycolysis, Krebs cycle, chemiosmosis
(C)glycolysis, Krebs cycle, fermentation
(D)oxidation of pyruvate, Krebs cycle, fermentation
7.What is the role of oxygen in cellular respiration?
(A)to combine with carbon and electrons to form carbon dioxide
(B)to combine with protons and electrons to form water
(C)to remove carbon from glucose to form pyruvate
(D)to remove carbon from pyruvate to form alcohol
8.Where are the electron transport chain and ATP synthase located?
(A)in the cytosol
(B)on the outer membrane of the mitochondria
(C)on the inner membrane of the mitochondria
(D)in the mitochondrial matrix
9.Which of the following choices correctly describes the flow of electrons in cellular respiration?
(A)glucose → Krebs cycle → oxygen → NAD+
(B)glucose → NAD+ → electron transport chain → oxygen
(C)glucose → electron transport chain → pyruvate → oxygen
(D)glucose → NAD+ → electron transport chain → carbon dioxide
10.Cyanide is a poison that blocks the movement of electrons down the electron transport chain. Which of the following would be the most immediate result of cyanide poisoning?
(A)No pyruvate would be produced.
(B)No NADH would form.
(C)No ATP would be produced.
(D)Chemiosmosis would not occur.
Short Free-Response
11.NAD+ has an important role as an electron carrier in glycolysis.
(a)Identify the process of aerobic cellular respiration during which a cell replenishes its supply of NAD+.
(b)Identify the process of anaerobic respiration during which a cell replenishes its supply of NAD+.
(c)If a cell’s supply of NAD+ ran out, predict the effect that would have on glycolysis within that cell.
(d)Justify your prediction from part (c).
12.In eukaryotes, ATP synthase is found in the inner membrane of the mitochondria. In prokaryotes, which do not have mitochondria, it is found on infoldings of the cell membrane.
(a)Make a claim as to why ATP synthase is found on the cell membrane in prokaryotes.
(b)Justify your claim from part (a) using your knowledge of how ATP synthase works.
(c)Explain why ATP synthase would not be effective if it was found in the cytosol of a cell.
(d)Describe how the structure of the mitochondria allows for the formation of a proton gradient.
Long Free-Response
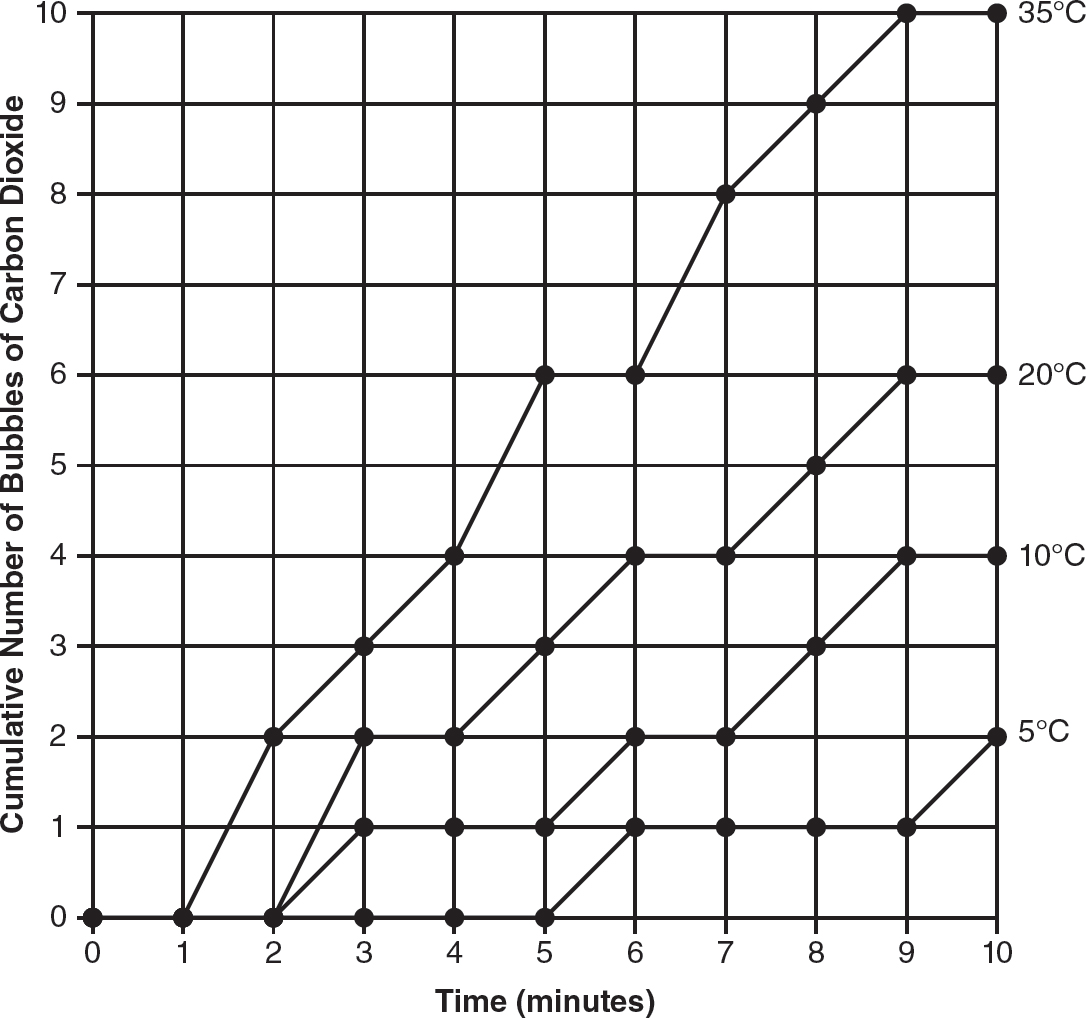
13.Saccharomyces cerevisiae (also known as baker’s yeast) is a eukaryotic organism. A solution containing 5% yeast and 10% glucose is placed in a transfer pipette. A weight is placed on the transfer pipette, and the apparatus is placed in water in a large test tube, as shown in the following figure.
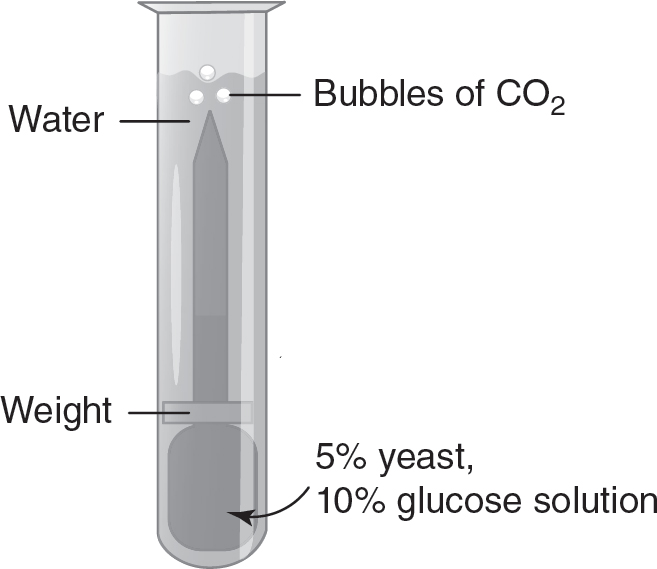
This process is repeated two more times, and each test tube is placed in a beaker of water at different temperatures (5° Celsius, 20° Celsius, and 35° Celsius). To measure the amount of cellular respiration, the cumulative number of bubbles of carbon dioxide released over a 10-minute period is recorded. The data are shown in the following table.
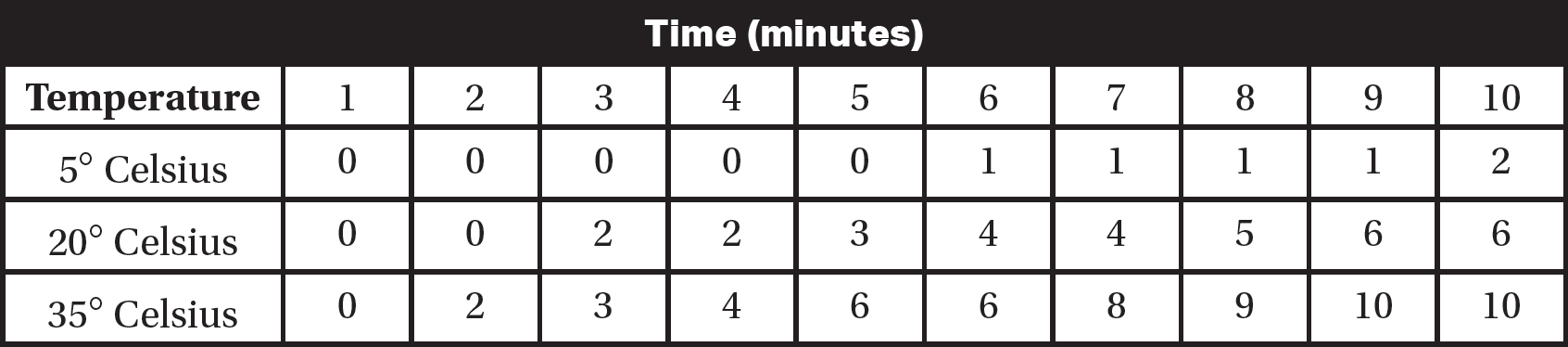
(a)Identify the independent variable and the dependent variable in this experiment.
(b)On the axes provided, construct an appropriately labeled graph of the experimental data.
(c)Calculate the average rate of carbon dioxide production per minute at each temperature.
(d)A fourth experimental apparatus is placed in a beaker of water at a temperature of 10° Celsius. Add a line to your graph from part (b) that predicts the rate of cellular respiration at 10° Celsius. Justify your prediction.
Answer Explanations
Multiple-Choice
1.(C)Fermentation oxidizes NADH into NAD+ so that NAD+ can be used in glycolysis. Choice (A) is incorrect because fermentation does not produce ATP. Pyruvate is a reactant used in fermentation, not a product of fermentation, so choice (B) is incorrect. While carbon dioxide is a product of alcohol fermentation, carbon dioxide is not always produced by fermentation (for example, lactic acid fermentation does not produce carbon dioxide). So choice (D) is incorrect.
2.(A)Glycolysis does not produce carbon dioxide. Choices (B), (C), and (D) all produce carbon dioxide, so all three of those choices are incorrect.
3.(D)Glycolysis does not produce a proton gradient; the movement of electrons down the electron transport chain produces a proton gradient. Choices (A), (B), and (C) are all true statements about glycolysis. Notice that this question was asking for the statement about glycolysis that is NOT correct, which is choice (D).
4.(C)Chemiosmosis uses a proton gradient to drive the production of ATP. If protons were able to pass freely through the inner mitochondrial membrane, a proton gradient could not be formed and chemiosmosis could not occur. Glycolysis (choice (A)), the Krebs cycle (choice (B)), and fermentation (choice (D)) do not require a proton gradient, so those processes would not be directly affected. Thus, choices (A), (B), and (D) are not correct.
5.(B)During oxidative phosphorylation, NADH is oxidized to NAD+ and oxygen is reduced to water. Choice (A) is incorrect because oxygen is not produced in oxidative phosphorylation. Choice (C) is incorrect because, during oxidative phosphorylation, NAD+ is not reduced and oxygen is not oxidized (it is reduced). Choice (D) is incorrect because NAD+ is not reduced and oxygen is not produced during oxidative phosphorylation.
6.(B)The vast majority of ATP produced in cellular respiration is produced by chemiosmosis (approximately 34 ATPs per glucose molecule!), and glycolysis and the Krebs cycle each produce a net gain of 2 ATPs per glucose molecule. Choice (A) is incorrect because the oxidation of pyruvate does not produce any ATP. Fermentation does not produce ATP, so choices (C) and (D) are incorrect.
7.(B)Oxygen is the final (or terminal) electron acceptor in cellular respiration; oxygen combines with electrons and protons to form water. While carbon dioxide is a product of cellular respiration, oxygen does not combine with carbon during cellular respiration to produce carbon dioxide. Thus, choice (A) is incorrect. Choice (C) is incorrect because oxygen neither removes carbon from glucose nor does it form pyruvate during cellular respiration. Removing a carbon from pyruvate to form an alcohol is what happens during fermentation, not cellular respiration, so choice (D) is incorrect.
8.(C)The electron transport chain and ATP synthase are both located in the inner membrane of the mitochondria. Choices (A), (B), and (D) do not list the correct location and are therefore incorrect.
9.(B)In cellular respiration, the electrons in glucose are transferred to NAD+ to form NADH. NADH then delivers these electrons to the electron transport chain. At the end of the electron transport chain, oxygen accepts these electrons (along with protons) to form water. Choice (A) is incorrect because the final electron acceptor in cellular respiration is oxygen, not NAD+. In cellular respiration, pyruvate is formed prior to electrons entering the electron transport chain, so choice (C) is incorrect. Choice (D) is incorrect because carbon dioxide does not receive electrons during cellular respiration.
10.(D)If electrons could not travel down the electron transport chain, a proton gradient would not be produced and chemiosmosis could not occur. Choice (A) is incorrect because the production of pyruvate does not require the electron transport chain. Choice (B) is incorrect because the ETC regenerates NAD+, not NADH. Limited amounts of ATP are produced in glycolysis and the Krebs cycle, so choice (C) is also incorrect.
Short Free-Response
11.(a)In aerobic cellular respiration, a cell’s supply of NAD+ is replenished during oxidative phosphorylation (when NADH delivers its electrons to the electron transport chain).
(b)In anaerobic respiration, a cell’s supply of NAD+ is replenished during fermentation (when NADH donates its electrons to a pyruvate molecule).
(c)If a cell’s supply of NAD+ ran out, the cell would not be able to undergo glycolysis.
(d)Glycolysis requires the input of NAD+; if there were no NAD+ available, glycolysis would halt. Living organisms require the input of energy, so the cell would eventually die.
12.(a)The enzyme ATP synthase is found on the infoldings of the cell membrane in prokaryotes because the enzyme needs a proton gradient to function.
(b)ATP synthase uses a proton gradient to drive the synthesis of ATP through chemiosmosis. To create a proton gradient, there must be a way to separate the protons. In eukaryotes, this separation occurs on either side of the inner mitochondrial membrane. Since prokaryotes do not have mitochondria, they separate protons across the only membrane they have, the cell membrane. So that is where ATP synthase is located in prokaryotes.
(c)If ATP synthase was found in the cytosol of the cell, there would be no way to separate the protons and create a proton gradient in the cytosol. So ATP synthase could not function.
(d)The mitochondria contain outer membranes and inner membranes. The electron transport chain is located on the inner membrane, and it pumps protons into the intermembrane space between the two membranes. ATP synthase, which is also located on the inner membrane, can then use this proton gradient to power the production of ATP.
Long Free-Response
13.(a)The independent variable is temperature. The dependent variable is the cumulative number of bubbles of carbon dioxide produced.
(b)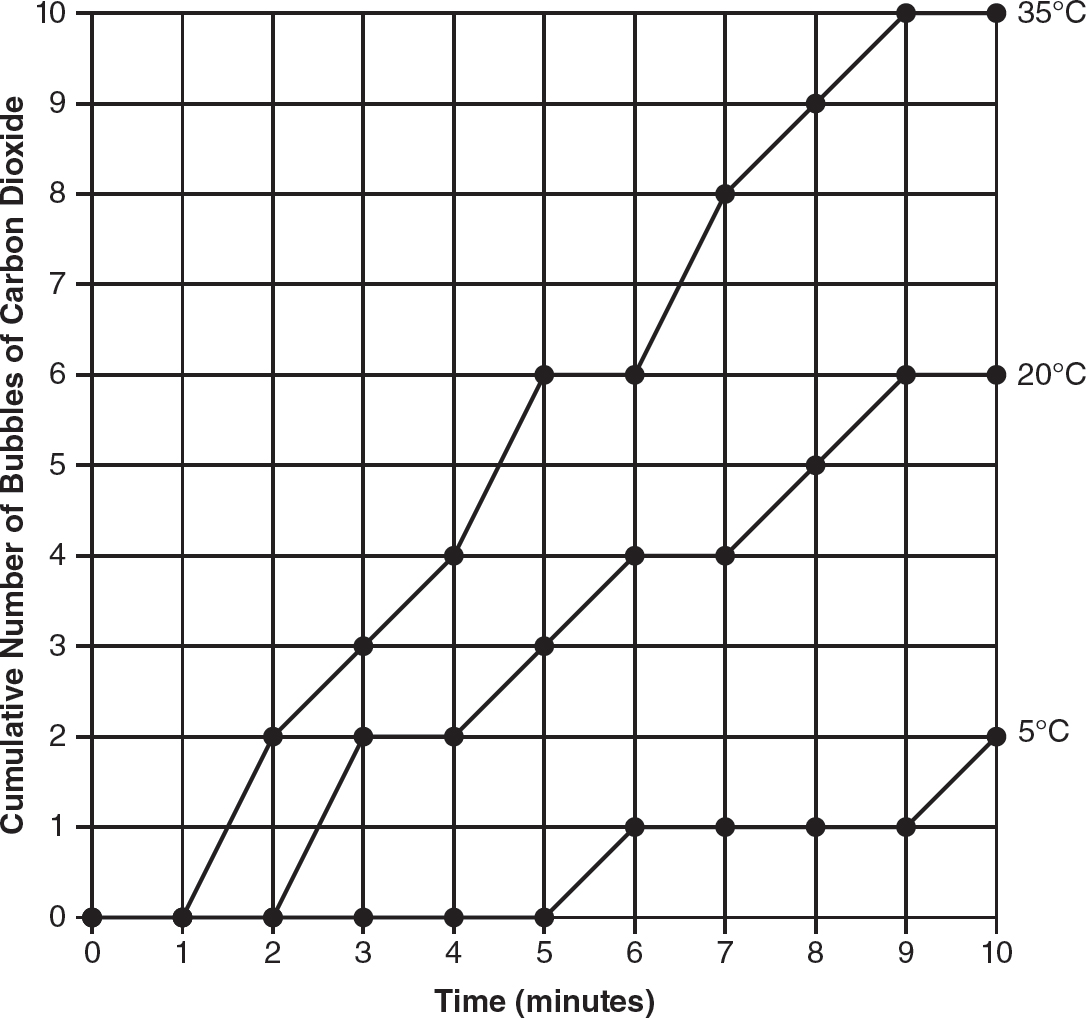
(c)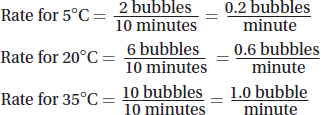
(d)The line for 10°C should be between the lines for 5°C and 20°C (higher than the line for 5°C and lower than the line for 20°C). This is because the rate of cellular respiration at 10°C will be higher than the rate at the colder temperature of 5°C and lower than the rate at the warmer temperature of 20°C.