Botany: An Introduction to Plant Biology - Mauseth, James D. 2017
Plant Physiology and Development
Energy Metabolism: Respiration

Chapter Opener Image: Plant tissues carry out aerobic respiration just as we animals do, and if submerged in water, they will drown. The roots of these bald cypress trees (Taxodium distichum) grow in mud below calm water, a region with almost no oxygen (bacteria use any oxygen that happens to diffuse down that far). But the roots send up “knees” that have thousands of air channels that permit oxygen to diffuse downward from the atmosphere to the roots. As long as the knees protrude above the surface of the water, the roots will have enough oxygen.
OUTLINE
✵ Types of Respiration
- Anaerobic Respiration
- Aerobic Respiration
- Heat-Generating Respiration
- Pentose Phosphate Pathway
- Respiration of Lipids
- Photorespiration
✵ Environmental and Internal Factors
- Temperature
- Lack of Oxygen
- Internal Regulation
✵ Total Energy Yield of Respiration
✵ Respiratory Quotient
✵ Fermentation of Alcoholic Beverages
- Beer
- Wine
- Spirits
- Warnings
Box 11-1 Plants and People: Fungal Respiration
Box 11-2 Alternatives: Respiration in Prokaryotes
Box 11-3 Plants Do Things Differently: Plants, Babies, and Heat
Box 11-4 Plants and People: Respiration
LEARNING OBJECTIVES
After reading this chapter, students will be able to:
✵ Explain what becomes of the excess energy produced by photosynthesis.
✵ Compare aerobic and anaerobic respiration.
✵ List the three parts of aerobic respiration.
✵ Identify a benefit of thermogenic respiration.
✵ Describe the value of the pentose phosphate pathway.
✵ Explain the relationship between temperature and respiratory rate.
✵ Describe how aerobic respiration is more efficient than anaerobic respiration.
✵ Define the respiratory quotient.
✵ Compare the fermentation processes of beer, wine, and spirits.
 Did You Know?
Did You Know?
✵ A glucose molecule has too much energy to be used in most chemical reactions; respiration breaks its energy into 36 smaller “pieces,” each in an ATP molecule.
✵ Respiration is one of the most ancient of all metabolisms: Almost all organisms respire by more or less the same metabolic pathways.
✵ Aerobic respiration requires oxygen and produces many ATP molecules for each glucose molecule respired.
✵ Anaerobic respiration does not require oxygen but produces fewer ATP molecules per glucose molecule.
✵ “Aerobic exercise” activities are actually anaerobic if you run, bike, or exercise so rapidly your blood cannot bring oxygen as quickly as your muscles need it.
![]() Concepts
Concepts
The light-dependent reactions of photosynthesis produce an excess of energy and reducing power, both of which are stored as glucose and starch; an important corollary must be the ability to recover that energy and reduced carbon. Recovery may occur later, when photosynthesis is impossible, such as night or in winter when the plant is leafless. Also, energy recovery may occur at a different site from photosynthetic capture: Glucose may be converted to sucrose, transported to apical meristems, vascular cambia, or any other heterotrophic tissue, then broken down to recover the energy (FIGURE 11-1).
Respiration is the process that breaks down complex carbon compounds into simpler molecules and simultaneously generates the adenosine triphosphate (ATP) used to power other metabolic processes (FIGURE 11-2). During respiration, carbon is oxidized. Its oxidation state goes from +0 to +4 as electrons are removed by NAD+, which is converted to NADH in the process. This is basically the opposite of photosynthesis, in which NADPH carries electrons to carbon, reducing it.
The NADH generated by respiration is a good reducing agent, but it is produced in much larger quantities than needed for constructive reduction reactions. Plants use mostly NADPH from photosynthesis for their reductions, not NADH, and most of the compounds that animals and fungi consume are already reduced. Because NADH contains a great amount of energy, it is selectively advantageous for an organism to be able to oxidize it so as to generate even more ATP.
Oxidation of NADH to NAD+ requires transferring electrons from it onto something else. The ideal recipient would be abundant, cheap, and nontoxic after it is used (FIGURE 11-3). These are also the three ideal characteristics of the source of electrons in photosynthesis, and the chemical involved in both processes is water. As it turns out, the result of respiration is the reverse of photosynthesis. Electrons are transferred from carbon in carbohydrate by means of reduced NADH, which carries them to an electron transport chain, which in turn deposits them onto oxygen, reducing it. As electrons are added, protons are attracted and incorporated, converting oxygen to water. If this reverse analogy were carried one step further, the electron transport chain would have to give off light, but that would be a complete waste of energy; instead, it conserves the energy as high-energy phosphate-bonding orbitals of ATP.
Respiration is also important to the plant because numerous intermediate compounds of its many steps are useful as starting materials for several anabolic pathways. A molecule of glucose, after entering the respiratory pathway but before being broken down to carbon dioxide and water, may be picked up in the form of an intermediate by a nonrespiratory enzyme that diverts it into a pathway that produces amino acids, fats, nucleic acids, lignin, and other molecules.
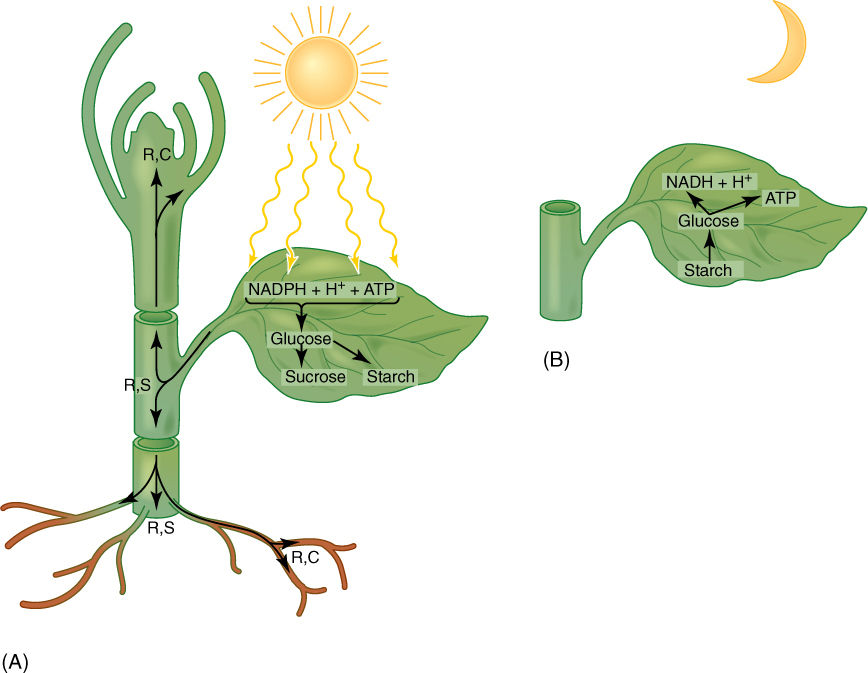
FIGURE 11-1 (A) When light is available, autotrophic tissues produce more ATP and NADPH than needed for cell metabolism. Glucose is produced, part of which is stored as starch in the leaf and part converted to sucrose and transported to areas that are either completely heterotrophic or are growing more rapidly than their own photosynthesis can support. Sucrose is converted back to glucose and is either respired (R) or used in the construction (C) of cell structures such as cellulose, lignin, amino acids, and nucleic acids. In roots, trunks, fruits, and seeds, some glucose may be polymerized to starch and stored (S) for months or years. (B) Even autotrophic tissues are heterotrophic in the dark, surviving on respiration of stored starch.
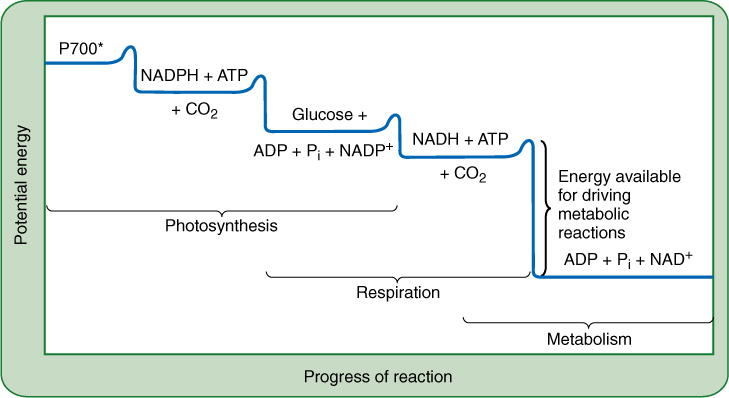
FIGURE 11-2 This hypothetical reaction diagram shows the relative levels of potential energy of the major compounds of photosynthesis and respiration. Energized P700 has the greatest potential energy, most of which is trapped in the formation of ATP and NADPH. When these reduce carbon dioxide and make glucose, much of the energy is conserved. Respiration transfers the energy to ATP and NADH. During other metabolic reactions, the breakdown of ATP and NADH yields large amounts of energy. Depending on the metabolic pathway, some of this energy is retained and some lost.
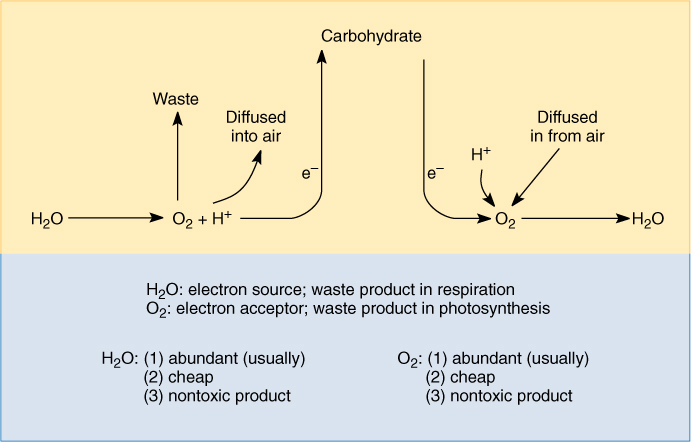
FIGURE 11-3 It is selectively advantageous for an organism’s metabolism to be based on raw materials that are abundant and cheap and produce nontoxic waste products. Mutations that cause an individual to require rare or expensive compounds or those that break down into toxic products are selectively disadvantageous.
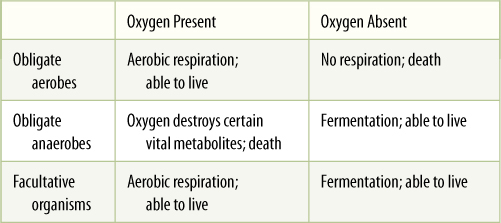
TABLE 11-1 Relationships Between Types of Organisms and Presence or Absence of Oxygen
![]() Types of Respiration
Types of Respiration
Cellular respiration falls into two categories: aerobic and anaerobic. Respiration that requires oxygen as the terminal electron acceptor is aerobic respiration. Under certain conditions, oxygen is not available, and an alternative electron acceptor must be used. This is anaerobic respiration, respiration without oxygen, often called fermentation. Because animals and plants must have oxygen for their respiration, they are known as obligate or strict aerobes (TABLE 11-1). At the opposite extreme are certain bacteria called obligate anaerobes, which carry out anaerobic respiration exclusively; such bacteria are actually killed by oxygen. Many fungi and certain types of tissues in animals and some plants are facultatively aerobic (or facultatively anaerobic): If oxygen is present, they carry out aerobic respiration, but when oxygen is absent or insufficient, they switch to anaerobic respiration. Although many fungi, especially yeasts, can live indefinitely anaerobically, plant and animal tissues can survive this way for only a short time. They must eventually obtain oxygen and switch back to aerobic respiration or they die.
Anaerobic Respiration
Glucose is broken down during anaerobic respiration by a metabolic pathway called glycolysis or the Embden-Meyerhoff pathway. Glycolysis and gluconeogenesis are essentially the same pathway, with the reactions running in opposite directions (FIGURE 11-4). Although all intermediates are the same, as are many of the enzymes, the two processes use different enzymes at certain key steps. This allows a cell to regulate the two processes so that one is stopped while the other runs; it would be useless for both pathways to operate simultaneously within a single cell.
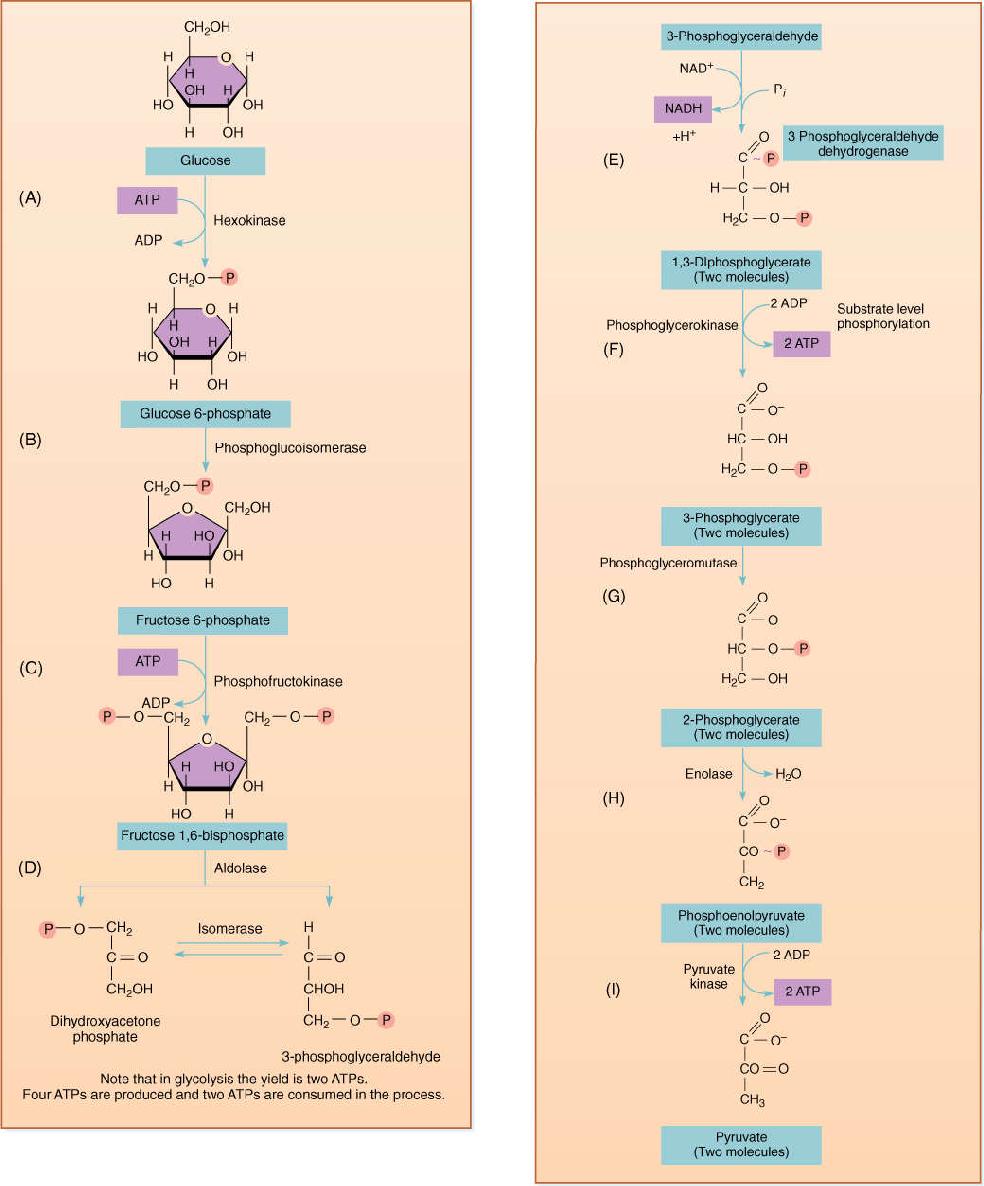
FIGURE 11-4 Glycolysis, also called the Embden-Meyerhoff pathway, constitutes the major portion of anaerobic respiration and is also the first part of aerobic respiration.
In glycolysis, ATP phosphorylates glucose to glucose-6-phosphate, which is then converted to fructose-6-phosphate. A second molecule of ATP then phosphorylates this to fructose-1,6-bisphosphate, which breaks down into 3-phosphoglyceraldehyde and dihydroxyacetone phosphate. The latter can be converted into a second molecule of 3-phosphoglyceraldehyde, and both can be oxidized to 1,3-diphosphoglycerate. During this oxidation step, electrons are transferred from a carbon of 3-phosphoglyceraldehyde to NAD+, converting it to NADH (as with NADPH, to be strictly correct when balancing equations, NADH should be considered to be associated with a proton: NADH + H+). The 1,3-diphosphoglycerate is energetic enough that an enzyme can transfer one of its phosphate groups onto an ADP, converting it to ATP and changing the 1,3-diphosphoglycerate into 3-phosphoglycerate, a process called substrate-level phosphorylation. The enzyme is phosphoglycerate kinase; the kinases constitute a large group of enzymes that remove phosphate groups from substrates. Phosphorylases are just the opposite, adding phosphates to substrates.
Although this is basically a reversal of the stroma reactions, ribulose-1,5-bisphosphate does not occur next as one might expect. Instead, 3-phosphoglycerate is converted first to 2-phosphoglycerate and then to phosphoenolpyruvate (PEP), the same metabolite that is the carbon dioxide acceptor in C4 metabolism and crassulacean acid metabolism (CAM). PEP is also energetic enough that an enzyme can transfer its phosphate group onto ADP to make ATP; dephosphorylation causes PEP to become pyruvate.
If no oxygen is present, anaerobic respiration has now removed all the energy possible. From each molecule of glucose, four ATPs were generated, and two were consumed; thus, there is a net production of two ATPs. Occasionally, the cell can start with glucose-6-phosphate and use one less ATP to get started. The ATP generated in anaerobic respiration is used for other metabolic reactions such as protein synthesis, nucleic acid replication, microtubule assembly, and ion transport. Indeed, these metabolic pathways are the reasons for respiration, and the ADP they generate migrates back to the sites of glycolysis and is rephosphorylated to ATP.
The reduction of NAD+ to NADH during glycolysis is a problem. If the cell needs reducing power, the NADH can be used, and it regenerates the NAD+ necessary to keep glycolysis running. For example, roots absorb nitrates and sulfates that must be reduced, and during the synthesis of fatty acids, large amounts of reducing power are needed. Many of these reductions can be carried out with this extra NADH (FIGURE 11-5), but usually a cell does not need as much reducing power as is produced during respiratory ATP production; consequently, NADH accumulates; all the NAD+ is consumed, and glycolysis stops for lack of NAD+. Without further glycolysis, no ATP would form and death would result. The big problem is converting NADH back to NAD+ by dumping its electrons onto something. In animal tissues under anaerobic conditions, the electron acceptor is pyruvate: NADH reacts with it to form lactate, the anion of lactic acid (FIGURE 11-6). In plants and fungi, pyruvate is first converted to acetaldehyde, and then NADH reacts with that, forming ethanol (ethyl alcohol; FIGURE 11-7A). Anaerobic conditions occur in plants and fungi growing in mud beneath stagnant water, especially in swamps and marshes (FIGURE 11-7B). Rice seeds germinate and grow anaerobically until the shoots reach oxygenated water (FIGURE 11-8).
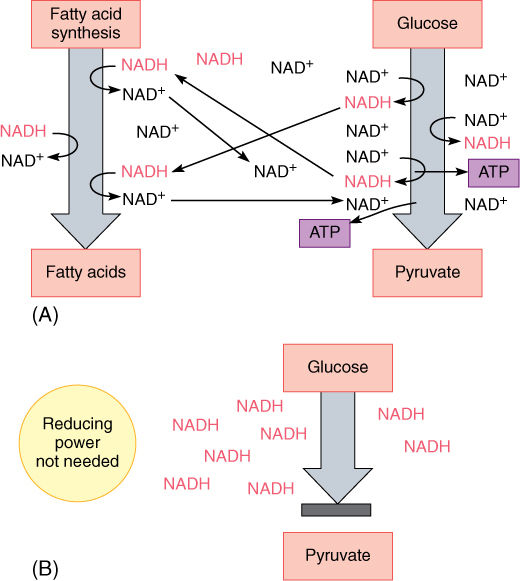
FIGURE 11-5 (A) If a cell needs reducing power, as for the synthesis of fatty acids, those reactions are actually electron-accepting reactions and they regenerate NAD+, which diffuses to other parts of the cell, allowing the continued production of ATP by glycolysis. (B) If reducing power is not needed, NAD+/NADH accumulates as NADH. Without NAD+, glycolysis stops: 3-phosphoglyceraldehyde cannot be oxidized to 1,3-diphosphoglycerate, and no ATP can be formed.
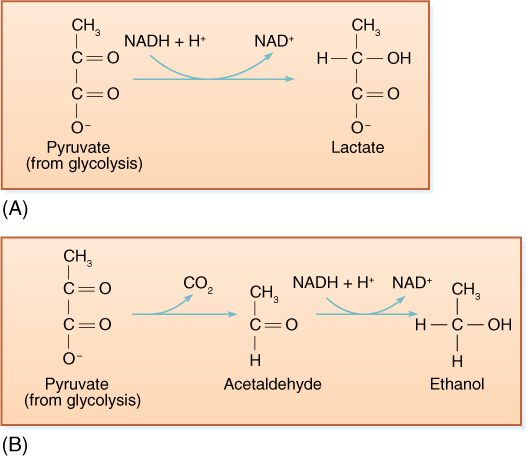
FIGURE 11-6 (A) When we exercise slowly enough for blood circulation to keep up, all our muscular activity is aerobic, but with rapid, intense, and prolonged activity, blood does not carry oxygen to the muscles rapidly enough and lactic acid fermentation begins. Lactate accumulation causes cramps and muscle pain. (B) Alcoholic fermentation involves the conversion of pyruvate to acetaldehyde before reduction by NADH + H+. Some goldfish survive low oxygen by respiring glucose to ethanol, for example, when ice on a lake surface prevents oxygen from dissolving into the water.
Plants and People
BOX 11-1 Fungal Respiration—The Prehistoric Industrial Revolution
Most college students know at least a little about fermentation—you probably already know that beer and wine are fermented even though you never much worried about the Embden-Meyerhoff pathway. Perhaps you know already that aerobic respiration of the fungi known as yeast causes bread to rise and holes to form in Swiss cheese, but you may not have realized that the use of these fermentations started out as lifesaving processes several thousand years ago.
When we think about the origins of agriculture between 2000 and 3000 BCE, we often think of the domestication of wheat, rice, and corn, but the use of fruits and animal products was necessary not only for diversity in the diet but also for diversity of vitamins and essential nutrients. Think about life in 3000 BCE—no supermarkets, of course, and no canned food, frozen food, or refrigerated food. People grew their own food, or collected it, or traded for it.
Now imagine the first cold winds and rains of autumn. How do you preserve the abundant food from summer to allow your survival through winter? It will be 7 long months before grains can be planted, before the dormant buds on grape vines open and put out their new leaves and flowers. Even after spring, the first crops cannot be harvested until May or June at the earliest. Hopefully, cows will give birth and there will be milk (cows lactate only while they have calves too young to eat grass). How do you preserve food for the winter?
Grains such as wheat dry naturally just before harvest and can be stored rather easily; they are so dry that microbes cannot grow. One problem is that they must be kept dry—protected not only from winter’s dampness but also from the water produced by their own slow respiration.
Fruits also can be dried, but these cannot be kept dry reliably without cellophane or plastic bags. Under ancient storage methods, dried fruit often drew enough moisture from wet winter air to allow fungi and bacteria to grow on them. One way to prevent microbial growth was discovered early: fermentation. If sugary fruits were partially respired in a sealed jar (anaerobic conditions), production of ethanol would finally kill all microbes, sterilizing the fruit. As long as the jar remained sealed, the fruit (or fruit juice) was safe, including all of its minerals, vitamins, amino acids, and so on. Beer is most often fermented barley, and barley grows best in cool, damp, northern climates where it is most difficult to keep grains dry.
Natural, unpasteurized milk becomes sour in just 2 or 3 days at room temperature (and, of course, in 3000 BCE just about all temperatures were room temperature) as bacteria grow rapidly, but if the right fungi are added, milk is only partially degraded and the resulting cheese is dry and stable enough to stop bacterial growth. Covered with a layer of wax to protect it from air-borne bacteria, it can last for years.
Using yeast to leaven bread (to make it rise and be spongy) has basically the opposite effect. If wheat is ground to flour, mixed with water, and then baked, the resulting bread can be so hard and dry that it will last forever, even in your mouth—mastication (chewing) is an ordeal. However, carbon dioxide from aerobic respiration of baker’s yeast becomes trapped by the bread dough, causing the bread’s texture to be lighter and more open. This bread does not preserve as well, but it is easier to eat.
Ethanol and lactate are not especially good solutions to the problem of NADH accumulation. The pyruvate consumed is always present in adequate amounts, of course, because glycolysis itself produces it, but it is not really “cheap” because many of its bonding orbitals have high-energy electrons. Furthermore, pyruvate could be used as a monomer for many types of synthesis. A lack of oxygen forces a cell to use a very valuable molecule as an electron dumping ground. Even worse, the products, either lactate or ethanol, are toxic. If they accumulate in the tissues or environment, they damage or even kill the cells producing them (TABLE 11-2).
TABLE 11-2 Electron Acceptors

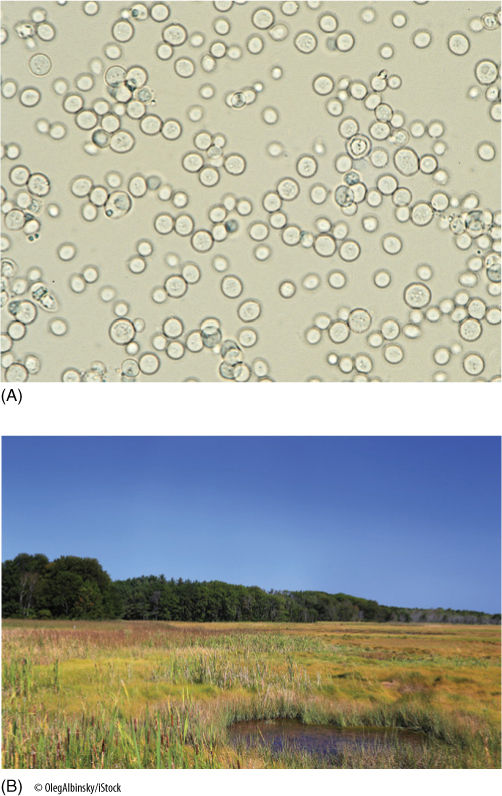
FIGURE 11-7 (A) In wine-making, yeast cells (Saccharomyces) ferment the glucose present in grapes and excrete the waste product ethanol. Naturally fermented wine has a maximum alcohol content of 14%; at that point, the waste product kills the yeast. Beer has a lower alcohol content, 5.5%, not because its yeast is more sensitive to the ethanol but because it is the legal limit. Fermentation must be stopped artificially, usually by heating the beer to kill the yeast. Alcoholic beverages with alcohol contents higher than 14% have extra alcohol added or part of their water removed (× 430). (B) The leaves and upright stems of these marsh plants exist in a highly oxygenated aerobic environment, but the rhizomes and roots exist in an anaerobic muck. Aerenchyma tissues in the stems permit the diffusion of some oxygen from leaves to rhizomes and roots, but some anaerobic respiration may be occurring. The trees in the background only survive where there is adequate oxygen in the soil.
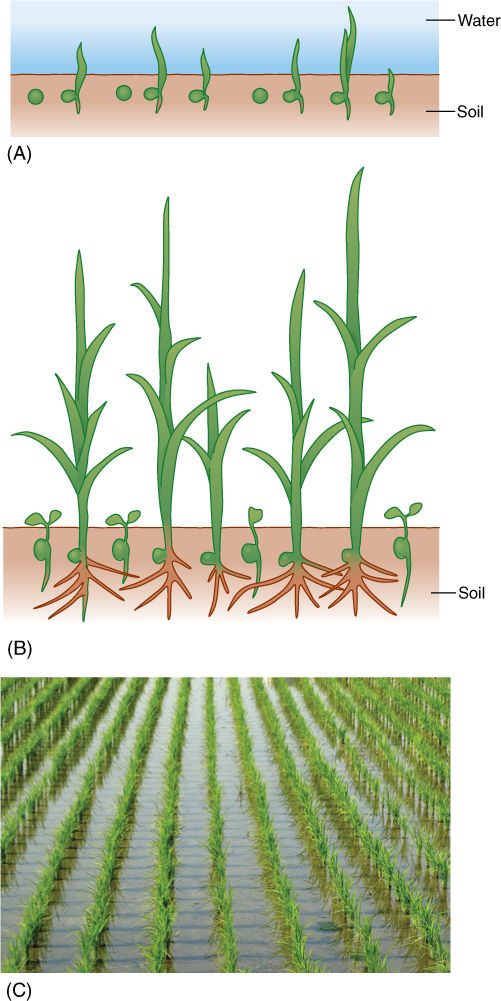
FIGURE 11-8 (A) Even under flooded, anaerobic conditions, rice seeds germinate and begin to grow because their embryos carry out facultative anaerobic respiration. Other seeds (round) remain dormant because they are incapable of fermentation; being dormant, they have a low rate of metabolism and the small amount of oxygen present may keep them from dying immediately. (B) When flooding subsides, oxygen is available, and nonrice seeds germinate; however, they are shaded by rice plants, and the soil is already filled with rice roots. The rice plants have a tremendous advantage. (C) Rice seedlings, still flooded.
Considering the negative aspects of anaerobic respiration, how could natural selection have produced something so inefficient? At the time life arose and respiratory pathways were evolving, Earth’s atmosphere contained reduced, hydrogen-rich compounds but no oxygen. Consequently, pyruvate and acetaldehyde had to be used as electron acceptors. Millions of years later, photosynthesis based on chlorophyll a evolved, and oxygen was released to the environment as a result of the water-splitting involved. After free oxygen became relatively abundant, the mutations leading to aerobic respiration began to be selectively advantageous. Glycolysis must be one of the most ancient of all metabolisms; it occurs in all organisms, with no exceptions.
Even in the presence of an oxygen-rich atmosphere and aerobic respiration, anaerobic respiration still has some selective advantage. Although the method is far from ideal, it does allow certain organisms to survive in particular environments. For example, because rice seedlings are capable of anaerobic respiration, they germinate and establish themselves during times of floods when other plants or seedlings are suffocating (Figure 11-8). In the absence of oxygen, the alternative to anaerobic respiration is death (see Table 11-1). Although this metabolism is expensive for the rice, by the time the flooding subsides, rice plants are already well rooted, have several leaves, and outcompete other species whose seeds are just beginning to germinate.
Alternatives
BOX 11-2 Respiration in Prokaryotes
Basic Reactions
In all plants, animals, fungi, cyanobacteria, and most bacteria and archaea, the compounds used for respiration are organic—sugars, fats, amino acids, and so on; however, certain prokaryotes (lithotrophs) metabolize inorganic compounds and extract energy from them, examples being hydrogen, sulfur, iron, and nitrogen.
Electron Donors
Hydrogen respiration is easy to understand. The reaction 2H2 + O2 → 2H2O liberates two electrons and releases so much energy that it easily forces electrons onto NAD+, producing NADH. This then donates the electrons to an ordinary electron transport chain that produces ATP. Hydrogen sulfide (H2S) and thiosulfate (S2O3−2) can both be converted to sulfur (S0) and then to sulfate (SO4−2). These reactions release energy and electrons that either go into an electron transport chain or react directly to produce ATP. The end product is sulfuric acid, which is toxic like the ethanol produced in plant and yeast fermentation. As long as the environment absorbs the acid and keeps it dilute, the bacteria are unharmed. Thiobacillus thiooxidans grows well even at pH 2. Oxidation of ferrous iron (Fe+2) to ferric iron (Fe+3) releases an electron that can be used to form a single molecule of ATP (FIGURES B11-2A and 11-2B). Likewise, ammonium (NH+4) can be oxidized to nitrite (NO2−) and then to nitrate (NO3−), releasing six energetic electrons that can be used for ATP synthesis.
Electron Acceptors
Just as various prokaryotes use various reduced compounds as sources of electrons and energy, other prokaryotes use various highly oxidized compounds as electron acceptors. The most common is nitrate (NO3−), which is converted to either NO2− or N2. Sulfate also acts as an electron acceptor and is converted to hydrogen sulfide. These processes are the opposite of those described previously here, in which these same compounds were used as substrates for respiration because they could be oxidized. How can they act as a source of electrons one time but as acceptors another time? The key is the presence or absence of oxygen: Oxygen has such a strong tendency to absorb electrons that in its presence the balance between H2S and SO4−2 is shifted in favor of SO4−2. Without oxygen, the balance is shifted the other way.
Certain organic compounds act as electron acceptors when oxygen is not present; the process is fermentation. Bacteria ferment diverse substrates such as numerous sugars and organic acids, resulting in products like ethanol, lactate, propionate, acetate, acetone, methanol, and carbon dioxide. Fermentations of amino acids (e.g., bacteria decomposing animal bodies), and other compounds that contain nitrogen or sulfur are often called putrefactions and produce odiferous compounds: hydrogen sulfide (aroma of rotten eggs), isobutyric acid, cadaverine (from lysine), putrescine (from ornithine), and ammonia. Researchers are experimenting with methods to increase fermentation of cellulose and lignin to ethanol or methane to be used as fuel, but as you know, these are extremely inert, nonreactive compounds.

FIGURE B11-2A This stream in Colorado is in a natural condition with clean water.

FIGURE B11-2B This stream is located only a few miles from the other, but it is polluted with runoff from mining wastes. Iron-oxidizing bacteria use the ferrous iron as a substrate, respiring it to ferric iron and generating ATP. Virtually no other life occurs in such polluted conditions.
Integrated Metabolism
Relationships between photosynthesis, respiration, and carbon are simple but important. All photosynthetic plants are autotrophs. They take in carbon dioxide, reduce it to carbohydrate by photosynthesis (carbon fixation), transport it to another part of the plant, and then use most to build structures. They respire the rest to produce ATP. All animals are heterotrophs: Their food consists of reduced carbon compounds, and a small amount is used to construct animal tissues; however, the majority is respired as an energy source. All animals lack the Calvin cycle; no animal is able to fix carbon dioxide.
In prokaryotes, similar relationships hold and others also occur. Green bacteria, cyanobacteria, and some purple bacteria are photosynthetic (phototrophic) and have the Calvin cycle (they can fix CO2: they are autotrophic). They are therefore photoautotrophs like plants. Most other bacteria, like animals, take in organic substances and use them both for ATP generation and for polymer construction; these are conventional heterotrophs. Photosynthetic bacteria, however, commonly have food and light available, and most purple bacteria and green bacteria are photoheterotrophs: They absorb and use organic carbon for construction and use photosynthesis almost exclusively to generate ATP. Little or no photosynthetic energy is used to reduce NADP+ to NADPH because NADPH is not in great demand, because the Calvin cycle is unnecessary if carbohydrate is present in food.
Lithotrophs use carbon dioxide as their primary carbon source; they are lithotrophic autotrophs. Examples are the colorless (nonphotosynthetic) sulfur bacteria, hydrogen bacteria, nitrifying bacteria, and iron bacteria. As they oxidize hydrogen, sulfur, iron, and so forth, the energy goes to make ATP, part of which is used for regular metabolism and part to pump protons, creating a pH gradient that can be used to reduce NADP+ to NADPH. Their energy metabolism is somewhat like bacterial photosynthesis. Neither metabolism can produce a compound as energetic as activated chlorophyll.
A very few bacteria (Beggiatoa, Thiobacillus, and some others) are lithotrophic heterotrophs: They get their energy by oxidizing sulfur compounds and they take in organic compounds for structural uses. They do not use carbon dioxide, and apparently they lack the Calvin cycle.
The ability to live as a heterotroph offers great advantages. By taking in carbon in its reduced form, heterotrophs use all their respiratory or fermentative energy for growth and reproduction. Autotrophs, which must use energy to reduce carbon dioxide, seem to be at a disadvantage. Their one advantage is that an autotroph has probably never starved to death; carbon dioxide is always abundant enough to sustain the life of autotrophs. The same is not true for the food supply of heterotrophs, whether that food is organic or not; famine is a common occurrence not only for animals and fungi but also for bacteria.
Aerobic Respiration
With oxygen present, the problems of using pyruvate or acetaldehyde are eliminated; oxygen is absorbed and acts as the terminal electron acceptor. Oxygen is inexpensive because it is absorbed and distributed by molecular diffusion, which requires neither active transport nor ATP consumption. Oxygen is abundant in most situations, and the product of reduction is water, which is not only nontoxic but actually beneficial. Aerobic respiration in plants is almost identical to that in animals, but we must be careful when comparing plants to ourselves. Our cells consist almost entirely of protoplasm whereas plant cells consist mostly of a large central vacuole: A kilogram of muscle for example contains much more metabolically active protoplasm than does a kilogram of parenchyma. Consequently, a kilogram of plant tissue needs much less oxygen than does a kilogram of animal tissue. Earth’s atmosphere now is 21% oxygen, but most plants survive with no trouble even if we artificially lower the oxygen to 5%; many plants thrive even in air with only 2% oxygen. Under ordinary circumstances, herbs, shrubs, and trees growing in regular air have about 250 times more oxygen than they need for full aerobic respiration.
Aerobic respiration consists of three parts: (1) glycolysis, (2) the citric acid cycle, and (3) oxidative phosphorylation in an electron transport chain.
Glycolysis
The initial steps of aerobic and anaerobic respiration are identical: glycolysis by the Embden-Meyerhoff pathway to pyruvate (Figure 11-4). This produces ATP and NADH just as before, but with oxygen present, the NADH migrates to electron carriers that oxidize it back to NAD+, permitting glycolysis to continue. Glycolysis occurs in the cytosol and plastids.
The Citric Acid Cycle
Because pyruvate is not needed as an electron acceptor, it can be used in a number of metabolic pathways. One of the main pathways takes advantage of the large amount of energy remaining in pyruvate and rather than using it for its structure, breaks it down and generates more ATP. This one pathway has three names: the citric acid cycle, the Krebs cycle, or the tricarboxylic acid cycle. These names reflect different facts about the cycle. One of the intermediates is citrate, the anion of citric acid. Much of the pioneering work on this metabolism was carried out by Hans Krebs. Finally, several of the intermediates are tricarboxylic acids—that is, each has three carboxyl (-COOH) groups.
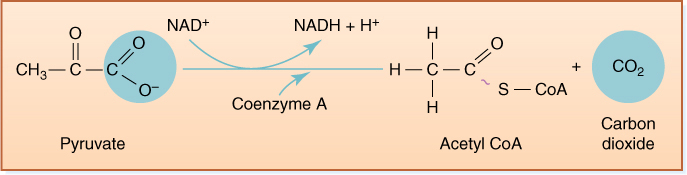
FIGURE 11-9 The process by which pyruvate is attached to CoA releases a carbon dioxide molecule and forms NADH. This step does not occur during anaerobic respiration; not only would it use up pyruvate needed as an electron acceptor, but it would generate even more NADH. Both of those results would be detrimental under anaerobic conditions, but both are beneficial when oxygen is present.
In the citric acid cycle, pyruvate is transported from the cytosol, where glycolysis occurs, across the mitochondrial membranes to the mitochondrial matrix. There it is oxidized and decarboxylated: As electrons are transferred to NAD+, bonding orbitals holding the last COO− rearrange and CO2 is liberated. Carbon dioxide and NADH are produced, along with a two-carbon fragment called acetyl (FIGURE 11-9). The carbon dioxide and NADH remain free in the matrix solution, but the acetyl becomes attached to a carrier molecule, coenzyme A (CoA), the resulting combination being acetyl CoA. Like pyruvate, acetyl CoA can be used in many synthetic pathways, but here we are interested in its entry into the citric acid cycle by transfer of the acetyl group to an acceptor molecule, oxaloacetate, a compound with four carbons (FIGURE 11-10). The oxaloacetate is converted to a six-carbon compound, citrate, which is then rearranged to cis-aconitate, which in turn is transformed to isocitrate (Figure 11-10, steps A, B, C, and D). In the next step, one of the carbons of isocitrate is oxidized by passing electrons onto NAD+, creating NADH. The oxidized carbon is liberated as carbon dioxide, leaving α-ketoglutarate, which has only five carbons (step E). This too is oxidized by NAD+, liberating another carbon dioxide, and the four-carbon remnant becomes attached to a new molecule of CoA in the process, forming succinyl CoA (step F). The energy released by the breakdown into free CoA, and free succinate can power phosphorylation of ADP to ATP (step G). The succinate still contains considerable energy and is oxidized to fumarate as electrons and protons are passed to flavin adenine dinucleotide (FAD), reducing it to FADH2 (FIGURE 11-11). Although the molecule becomes oxidized by this step, no carbon dioxide is lost; the fumarate is also a four-carbon compound. It reacts with a water molecule and becomes malate, which passes a final set of electrons onto NAD+ and is transformed into the original acceptor molecule, oxaloacetate (steps H, I, and J).
It was mentioned above that the benefit of the citric acid cycle is the generation of more ATP; nevertheless, at only one step has ATP been produced. Instead, there are four steps in which more NAD+ is reduced to NADH and one in which FAD is reduced to FADH2. Excess NADH and the related deficiency of NAD+ were seen to be problems in anaerobic respiration, and thus, the citric acid cycle at first seems to be a real contradiction. However, in the next step, the electron transport chain, the energy in NADH and FADH2 drives the synthesis of ATP, and NADH is simultaneously oxidized back to NAD+.
The Mitochondrial Electron Transport Chain: Chemiosmotic Phosphorylation
The mitochondrial inner membrane, like the chloroplast inner membrane, contains sets of compounds capable of carrying electrons (FIGURE 11-12). Although many of the actual carriers differ in the two organelles, the principles of electron transport are the same. The carriers that react with NADH or oxygen are placed precisely and asymmetrically in the crista membranes, both on the matrix side only (FIGURE 11-13). The exact order of carriers in plant mitochondria is complex, and numerous types exist. Only the most well-understood are presented later here.
NADH diffuses to the membrane and passes electrons to a protein that has flavin mononucleotide (FMN) bound to it as a cofactor. The FMN is reduced, and the NADH simultaneously oxidized to NAD+, which can migrate back to the site of the citric acid cycle. Reduced FMN (FMNH2) passes the electrons to a set of iron- and sulfur-containing, proteinaceous electron carriers, which transfer the electrons to one or several quinones, one of which is ubiquinone (also called coenzyme Q; FIGURE 11-14). From the pool of quinones, electrons are transferred to cytochrome b. The next carriers in sequence are more quinones, then cytochrome c1, cytochrome c, cytochrome a, and cytochrome a3. Cytochromes a and a3 are part of a large enzyme complex known as cytochrome oxidase, which contains several proteins and two copper ions that mediate transfer of electrons from the iron in cytochrome to oxygen. As oxygen is reduced, it picks up two protons and becomes water.
If this electron transfer were the only thing to happen in the electron transport chain, it would be better than anaerobic respiration because the cell could avoid the waste of pyruvate and the synthesis of the toxic lactate or ethanol, but much more is accomplished. Mitochondria also perform chemiosmotic phosphorylation. As with chloroplasts, the fate of the protons released and absorbed during electron transport is important. In mitochondria, when NADH reacts with FMN, two protons are transferred as well as electrons. One proton comes from the NADH and the other from the water, both on the matrix side of the membrane (a few water molecules are always splitting spontaneously [H2O → H+ + OH−]; water is not split specifically as in photosystem II of photosynthesis). When FMNH2 reduces any quinone, the two protons are released on the other side of the membrane into the lumen of the crista. This step of electron transport acts as a proton pump; a large concentration of protons begins to build up in the lumen while there is a lack of protons in the matrix. Ubiquinone and the other quinones just before cytochrome c1 act in a similar manner, pumping protons out every time they carry electrons. Also, as oxygen receives electrons from the electron transport chain, it forms water by picking up two protons from the matrix, decreasing the proton concentration. In a short time, a proton concentration gradient develops that is strong enough to cause the protons to migrate back into the matrix.
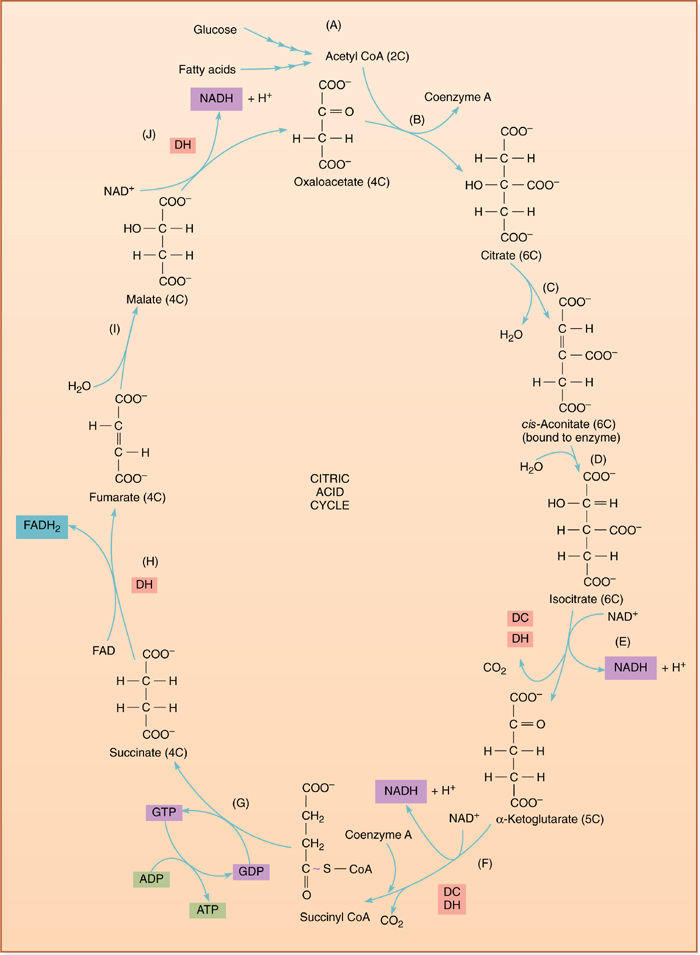
FIGURE 11-10 The steps of the citric acid cycle. This cycle is an important part of aerobic respiration, even though it does not consume oxygen and generates only one molecule of ATP directly. DH = dehydrogenation (oxidation); DC = decarboxylation.
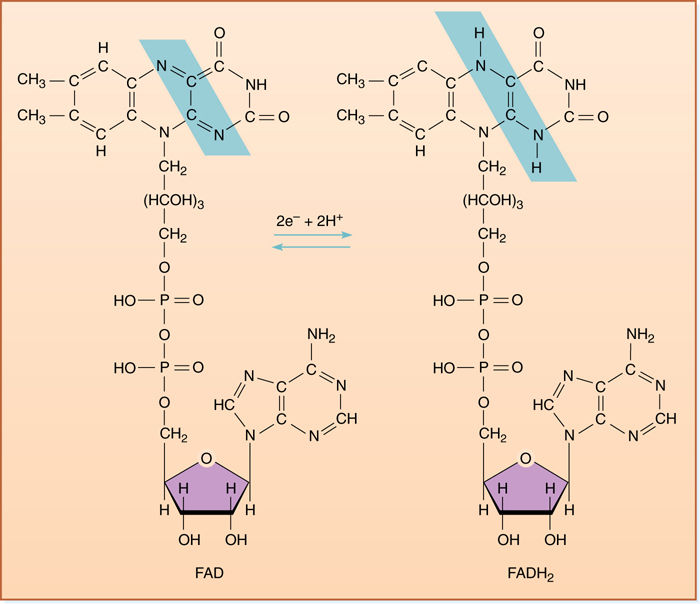
FIGURE 11-11 Flavin adenine dinucleotide (FAD) is a large organic electron carrier similar to NAD+. When it is reduced by two electrons, it picks up two protons, becoming FADH2. Unlike NADH + H+, both protons are covalently attached to FADH2 (shown in blue box).
The flow of protons from the crista lumen to the mitochondrial matrix can be used to synthesize ATP. As in chloroplasts, ATP synthetase channels in the membrane use the flow of protons to force a phosphate group onto ADP, creating ATP. This is a chemiosmotic phosphorylation, and because of it, the NADH is an excellent if indirect source of ATP rather than a problem. The electrons that each molecule of NADH contributes to the mitochondrial electron transport chain provide enough power to create three ATPs.
FADH2 also passes its electrons to the mitochondrial electron transport chain, but it reacts with ubiquinone instead of FMN; thus, the first step in proton pumping does not use these electrons. Therefore, FADH2 contributes less to the proton gradient than does NADH, providing enough power for production of two ATPs rather than three.
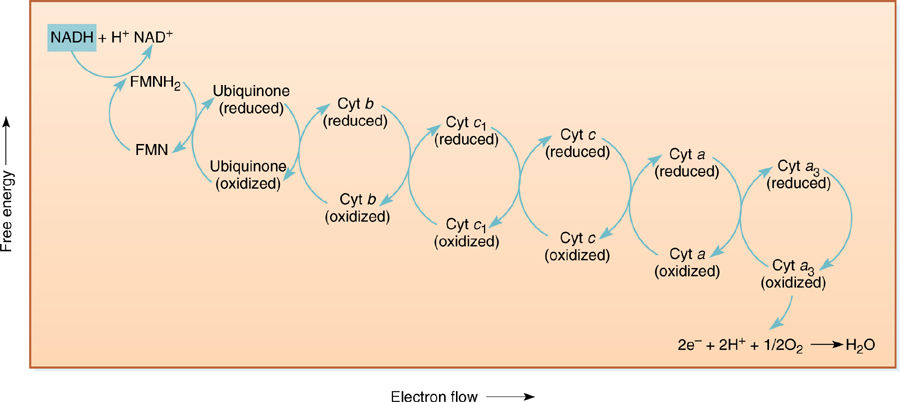
FIGURE 11-12 Membrane-bound electron carriers of the mitochondrial electron transport chain. As in chloroplasts, their positions and movements are important (see Figure 11-13). Electrons are brought to membrane-bound carriers by mobile carriers such as NADH and FADH2, which are produced in the matrix.
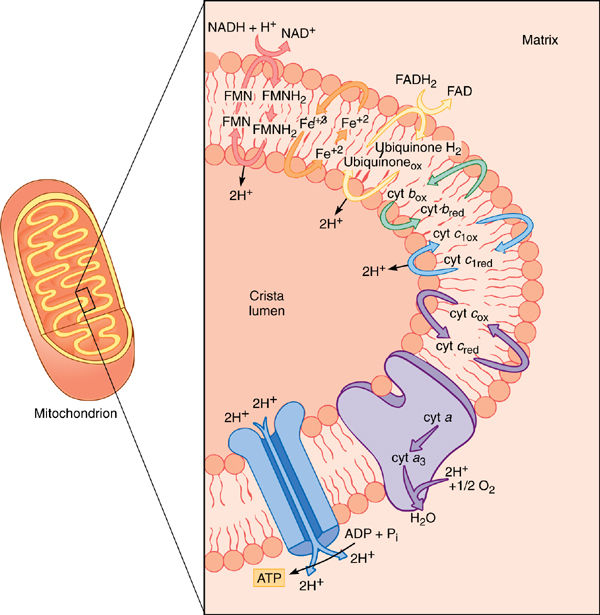
FIGURE 11-13 Only the important components of the mitochondrial electron transport chain are shown in this diagram—the steps that transport protons from the matrix to the crista lumen, establishing a proton/hydroxyl chemiosmotic gradient, just as in chloroplasts. The formation of water contributes to the gradient because protons are absorbed from the matrix but not from the cristae. FMN is flavin mononucleotide, an electron carrier similar in structure to FAD.
The NADH Shuttle
NADH produced by glycolysis cannot cross the mitochondrial inner membrane and donate electrons directly to the electron transport chain because the inner membrane is impermeable to such large molecules. Instead, a series of chemical reactions carries (shuttles) reducing power across the membrane (FIGURE 11-15). Several shuttle mechanisms occur. In the malate-aspartate shuttle, NADH in the cytosol powers the conversion of oxaloacetate to malate, which crosses to the mitochondrial matrix and powers the formation of a new molecule of NADH. Malate is converted to aspartate and transported back out of the mitochondrion, where it is converted to oxaloacetate again and can repeat the cycle. In this shuttle, each cytosolic NADH drives the formation of a matrix NADH and the consequent oxidative phosphorylation of three ADPs to three ATPs.
In a second type of shuttle, the glycerol phosphate shuttle, cytosolic NADH reduces dihydroxyacetone phosphate to glycerol phosphate, which is transported across the inner membrane to the matrix. There it converts back to dihydroxyacetone phosphate, reducing FAD to FADH2 in the process. Each cytosolic NADH results in the formation of one matrix FADH2, which can drive the formation of only two ATPs; not as much energy is conserved in this shuttle.
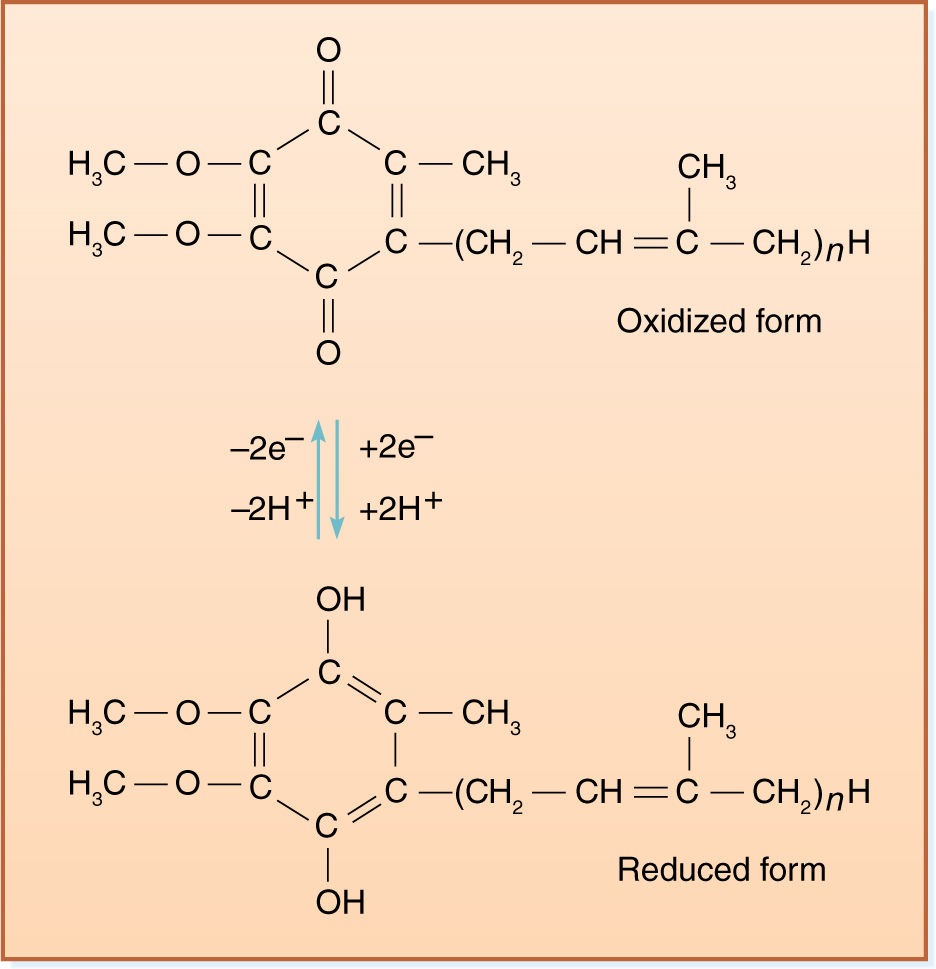
FIGURE 11-14 Ubiquinone (coenzyme Q) is a quinone that can carry two electrons and two protons simultaneously.
Plants Do Things Differently
BOX 11-3 Plants, Babies, and Heat
Most plants resemble cold-blooded animals in having a temperature close to that of the immediate environment and lacking thermogenic mechanisms. Plants are not known to have any ability to sense their own temperature, and the few plant tissues and organs that do heat up do so in response to their own developmental state, such as being ready to open their flowers.
In contrast, warm-blooded animals like us humans monitor our internal body temperature—core temperature as opposed to skin temperature—by the hypothalamus portion of our brain. It can detect changes in core temperature of as little as 0.01°C, and when core temperature drops, the hypothalamus causes heat generation. A strange thing is that we warm-blooded animals do not have a direct method of thermogenesis: All we can do is shiver, rapidly contracting and relaxing our muscles and wasting ATP just for the heat released.
The few plants that generate heat do so more directly, using thermogenic respiration, which transports electrons in mitochondria without pumping protons and without saving any of the energy as ATP. Newborn human babies have a thermogenic mechanism rather similar to that of plants. They have a special type of fat, called brown fat, in which the cells are filled with numerous mitochondria, but these mitochondrial membranes are permeable to protons. Mitochondrial electron transport occurs, along with proton pumping; however, no chemiosmotic gradient builds up, and no ATP is synthesized. Thus, all energy is converted to heat. This is an extremely efficient method of generating heat, but for some reason, we give this up when we are several months old. While still babies, we acquire the ability to shiver and lose all of our brown fat. Mammals that hibernate, however, retain brown fat thermogenesis: Hibernating bears stay warm just like human babies, and almost like plants.
Brown fat cell and plant thermogenic respiration have similar results—the generation of heat. It is not known whether one is more suited to animals and the other to plants. Perhaps in animals the mutation of proton-permeable membranes occurred earlier than mutations of the genes for alternative carriers, whereas in plants the opposite occurred.
In at least some plants, NADH can cross the outer mitochondrial membrane and react directly with ubiquinone at the outer surface of the inner membrane. A shuttle mechanism is not necessary, but the step of proton pumping by FMN is bypassed, decreasing the amount of ATP that can be generated.
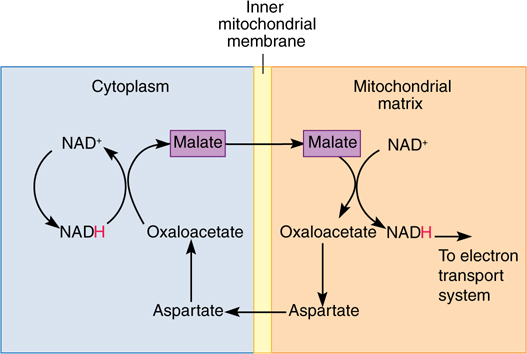
FIGURE 11-15 The malate—aspartate shuttle allows reducing power to be transferred into mitochondria when NADH itself cannot cross the mitochondrial membranes.
Heat-Generating Respiration
During glycolysis, the citric acid cycle, and mitochondrial electron transport, small amounts of energy are lost in each step even though a great deal of energy is conserved by the synthesis of ATP (see Figure 11-2). The total chemical energy of all products (ATP, carbon dioxide, and water) is less than that of all reactants (gluclose-6-phosphate and oxygen); the difference is “lost” as heat and increased entropy (disorder). For example, compost piles become warm because of the heat loss during the respiration of the fungi and bacteria that decompose the compost. Heat loss is usually an inefficient aspect of respiration; in most cases, it would be selectively advantageous for a plant to produce more ATP from each molecule of glucose respired and lose less heat to the environment.
The heat “lost” during respiration by warm-blooded mammals is vitally necessary to maintain body temperature, and when we are chilled, we must generate even greater amounts of heat by shivering: Our muscles contract and relax rapidly, breaking down large amounts of ATP and releasing the stored energy as heat.
Some plants also generate large amounts of heat. In the voodoo lily (Sauromatum guttatum), parts of the inflorescence become much warmer than the surrounding air, causing amines and other chemicals to vaporize and diffuse away as chemical attractants for pollinators. Skunk cabbage (Symplocarpus foetidus) often begins floral development while covered with snow; it melts the snow cover and exposes its flowers by generating large quantities of heat (FIGURE 11-16). These and other plants produce heat much more efficiently than humans do; they have alternative electron carriers that apparently do not pump protons during electron transport in mitochondria. Consequently, there is no proton gradient and no chemiosmotic production of ATP. The energy in NADH is converted entirely to heat, and the tissues become quite warm (FIGURE 11-17).

FIGURE 11-16 By generating heat internally, skunk cabbage (Symplocarpus foetidus) maintains a high enough temperature to carry out active metabolism even when covered by snow. When it is ready to emerge, it produces even more heat and melts the snow, revealing the inflorescence to pollinators.
In ordinary mitochondria, cyanide (CN−), azide (N3−), and carbon monoxide (CO) interfere with the last electron carrier, cytochrome oxidase. When they are present, electrons cannot pass from cytochrome c to oxygen; without electron flow and consequent ATP generation, both animals and plants die unless capable of anaerobic respiration. In plants that generate heat, the alternative electron carriers do not interact with cyanide, azide, or carbon monoxide, and heat is generated even if these chemicals are present. Heat-generating respiration can thus be studied by poisoning normal aerobic respiration with cyanide; consequently, heat-generating respiration is usually called cyanide-resistant respiration. A better name is thermogenic respiration.
The term “cyanide-resistant respiration” is somewhat misleading because it suggests that plants can be immune to cyanide poisoning. The analogy that because anaerobic respiration allows cells to survive in anaerobic conditions, cyanide-resistant respiration must allow cells to survive in the presence of cyanide is incorrect. Plants never encounter high concentrations of cyanide in natural conditions; if they did they would be killed because aerobic ATP generation is blocked. “Thermogenic respiration” and “heat-generating respiration” are less confusing terms.
Many aspects of thermogenic respiration are still unknown. The alternative carriers appear to be present in all mitochondria, and they seem to be resistant to stressful conditions such as cold or desiccation. One hypothesis is that during stress, the ordinary, proton-pumping carriers do not function as well, causing ATP production to drop, but the alternative carriers continue to transport electrons, allowing NADH to be converted back to NAD+, which can then be used to keep glycolysis running and producing ATP. If this hypothesis is correct, thermogenic respiration is a metabolism that typically functions only rarely and helps plants survive stress conditions, and it has been adapted for actually generating heat in a small number of species.
Pentose Phosphate Pathway
The intermediates of all respiratory pathways can be used in other pathways to make various compounds; it is not inevitable that they will be completely oxidized to carbon dioxide and water. The pentose phosphate pathway, which involves several intermediates that are phosphorylated five-carbon sugars (pentoses), is an important source for many fundamental compounds. It is usually included in discussions of respiration because it begins with glucose-6-phosphate, gives off carbon dioxide, and involves oxidations that produce NADPH (FIGURE 11-18); however, its importance as a source of respiratory energy is much less significant than its role as a synthetic pathway. The pentose phosphate pathway transforms glucose into four-carbon sugars (erythrose) and five-carbon sugars (ribose) that are essential monomers in many metabolic pathways. The ribose-5-phosphate produced can be shunted into nucleic acid metabolism, forming the basis of RNA (ribonucleic acid) and DNA (deoxyribonucleic acid) monomers, the nucleotides. In meristematic cells, large amounts of DNA must be synthesized during the S-phase of a short cell cycle; the pentose phosphate pathway is an extremely important part of the metabolism of these cells.
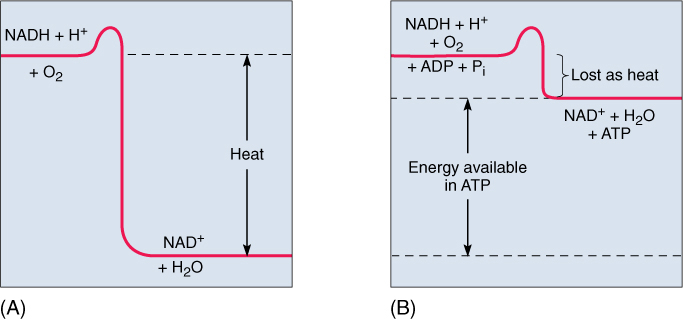
FIGURE 11-17 If NADH breaks down to NAD+, a large amount of energy is liberated. (A) If this powerful exergonic reaction is not coupled to any endergonic reaction, all of the energy is converted to heat. (B) When coupled to the endergonic synthesis of ATP from ADP, most of the energy is conserved and little is converted to heat.
The four-carbon sugar erythrose-4-phosphate is the starting material in the synthesis of many compounds. Two important types are lignin and anthocyanin pigments. Tissues such as wood, fibers, and sclereids deposit large amounts of lignin into their secondary walls during development, and erythrose-4-phosphate is in great demand. During differentiation, these cells use the pentose phosphate pathway but pull out erythrose-4-phosphate, not ribose-5-phosphate. Flower petals and brightly colored fruits divert erythrose-4-phosphate from the pentose phosphate pathway to anthocyanin production while synthesizing their pigments. The pentose phosphate pathway also occurs in plastids, where it supplies erythrose-4-phosphate for synthesis of amino acids such as tyrosine, phenylalanine, and tryptophan.
In addition to these four- and five-carbon sugars, the pentose phosphate pathway also produces NADPH. Although many anabolic reductions in the cytoplasm use NADH rather than NADPH, the reduction of nitrate (NO3−) to amino acids (NH3+) can be accomplished only by NADPH, which can be generated by the pentose phosphate pathway.
Many of the reactants in the pentose phosphate pathway are the same as those in glycolysis, and both pathways occur in the cytosol and plastids. They are best understood as interconnected and simultaneous pathways. In meristematic cells, the pentose phosphate pathway is active and shifted in favor of ribose production. In wood cells, it is also active but produces erythrose. In other cells, it may be much less active and glycolysis may dominate, producing NADH that then powers ATP production in mitochondria. Imagine a cell as it is produced in the vascular cambium (meristematic, needs nucleic acids) and then differentiates into lignified wood parenchyma (needs lignin), which after the wall is mature, takes on the role of storing starch during the summer and releasing it in the spring (FIGURE 11-19). Energy metabolism is adjusted at each stage in a major way, and smaller changes in the rates of reactions may occur on a day-to-day basis (FIGURE 11-20).
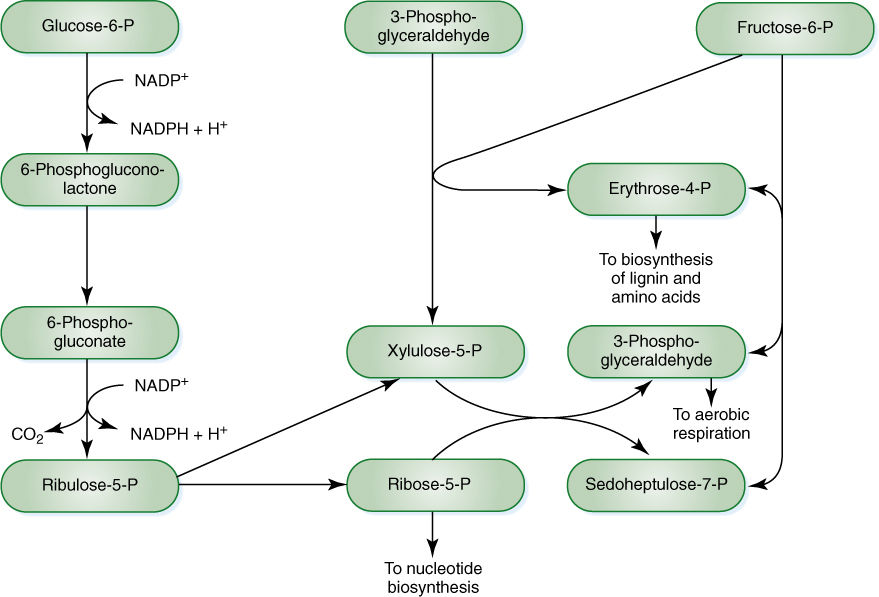
FIGURE 11-18 The pentose phosphate pathway, showing all intermediates. If ribose-5-phosphate is drawn off into nucleic acid metabolism, the pentose phosphate pathway is shifted in favor of ribose production. If erythrose-4-phosphate is diverted to lignin metabolism, the pentose phosphate pathway reaction equilibria are shifted toward erythrose production.
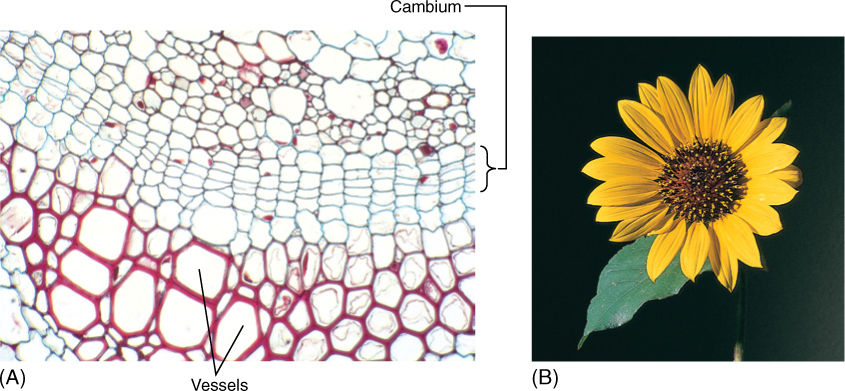
FIGURE 11-19 (A) At the cambium, the pentose phosphate pathway may be producing ribose-5-phosphate, but in the differentiating vessels, it is producing erythrose-4-phosphate. In all cells, glycolysis and the rest of aerobic respiration are occurring simultaneously with the pentose phosphate pathway (× 400). (B) In petals, erythrose-4-phosphate, used in the production of pigments, is produced by the pentose phosphate pathway.
Respiration of Lipids
Some tissues, especially oily seeds and dormant apical meristems, store large amounts of lipid, usually as triglycerides or phospholipids. During germination or release from dormancy, lipids undergo catabolic metabolism in which they are broken down into glycerol and three fatty acids (triglycerides) or glycerol phosphate and two fatty acids (phospholipids). Fatty acids are then further broken down into two-carbon units—acetyl CoA—by a process called β-oxidation in either cytosol or microbodies called glyoxysomes. For example, an 18-carbon fatty acid would be converted into nine acetyl CoA units. As each acetyl CoA is formed, one FAD is reduced to FADH2, and one NAD+ is reduced to NADH, both of which can carry electrons to mitochondria and drive production of ATP by means of the electron transport chain (FIGURE 11-21). Acetyl CoA may be used for synthesis of carbohydrates and other compounds, or it may enter the citric acid cycle and be further respired.
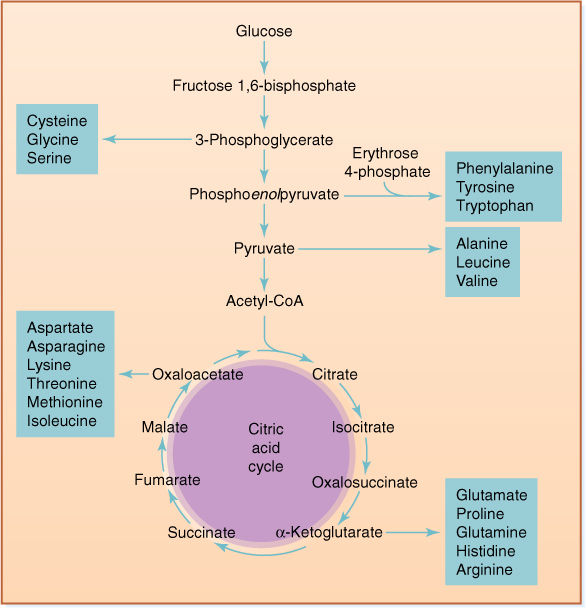
FIGURE 11-20 All 20 amino acids are constructed from intermediates of respiration. Although too much sugar harms our health, plants construct everything from glucose and some minerals.
Photorespiration
Photorespiration occurs only when RuBP carboxylase adds oxygen rather than carbon dioxide to ribulose-1,5-bisphosphate, resulting in one molecule of 3-phosphoglycerate and one of phosphoglycolate. Phosphoglycolate is dephosphorylated to glycolate, which is then transported to microbodies called peroxisomes. Glycolate can be converted to glycine in the peroxisomes, and the glycine may be transferred to mitochondria, where it is respired to carbon dioxide and water with no conservation of energy in either ATP or NADH; all energy is wasted. In some cases, some of the glycine can be converted to serine; both are useful amino acids. Other mechanisms also produce these amino acids, however, and direct measurements show that for many C3 species, photorespiration wastes as much as 30% of the energy trapped by photosynthesis.
![]() Environmental and Internal Factors
Environmental and Internal Factors
As with photosynthesis, numerous environmental factors influence the rate of respiration. It is necessary to consider the integration of respiration into the total biology of the plant as well as the metabolic pathways involved.
Temperature
Temperature greatly influences respiration in a plant growing under natural conditions. In all environments, shoots located in air are at a different temperature than roots in soil. Shoots are subjected to great changes of temperature from day to night, often more than 20°C.
In most tissues, an increase in temperature of 10°C, in the range between 5°C and 25°C, doubles the respiration rate, a magnitude of increase seen in many enzyme-mediated reactions. Below 5°C, respiration decreases greatly (FIGURE 11-22). Above 30°C, respiration still increases, but not so rapidly; at such high temperatures, oxygen probably cannot diffuse into tissues as rapidly as the tissues use it. Above 40°C, respiration, like many other processes, slows greatly, probably because of enzyme damage or disruption of organelle membranes.
Lack of Oxygen
Because plants are not as active as animals, much lower oxygen concentrations—as little as 1% to 2%—maintain full rates of plant respiration. Oxygen concentration in the atmosphere is so stable that it does not cause variations in respiration, but variations occur in a cell’s access to oxygen. During daylight hours, chlorophyllous tissues and organs produce oxygen, some of which is used in respiration. At night, oxygen is not produced, but it can diffuse into the large intercellular spaces of the plant if it can penetrate the closed stomata. Because the cuticle is not absolutely impermeable to oxygen and because the atmosphere contains such a high concentration, oxygen diffuses in rather well, even through closed stomatal pores. Less oxygen is available, but the temperature is lower, and thus, demand for oxygen is reduced.
Oxygen availability is much more variable for roots; in well-drained soil, the large quantity of gas located between soil particles is depleted in oxygen owing to respiration by roots, fungi, bacteria, protists, and soil animals. During and after rain, soil air is displaced by water, and roots either have lesser amounts of oxygen (hypoxia) or none (anoxia) available. Anaerobic respiration allows roots to survive for a short period, but it is not sufficient for root growth and healthy metabolism. If continually flooded, roots of almost any species die. Those that survive do so primarily by oxygen diffusion from the stems through aerenchyma channels in the cortex or pith, such as in petioles of water lilies and cattails. Floods that submerge the bases of shoots or woody trunks are especially damaging if they last more than a few days. Rice seedlings are the only certain example of plants that can grow normally without oxygen for a prolonged period.
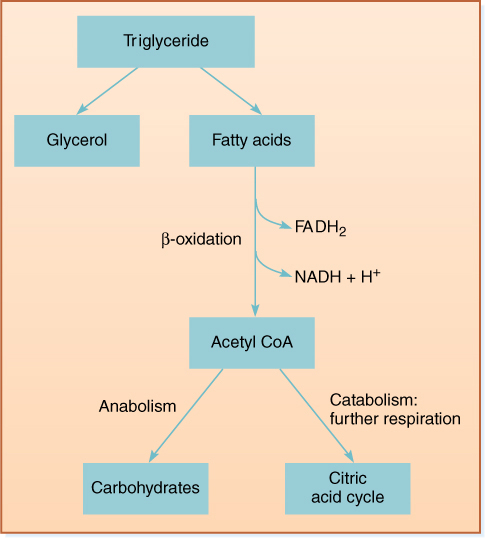
FIGURE 11-21 During the respiration of lipids, fatty acids are separated from glycerol and undergo β-oxidation to acetyl CoA units, producing FADH2 and NADH. In germinating seeds, much of the acetyl CoA is converted to glucose and fructose by gluconeogenesis then are polymerized into sucrose. The sucrose is transported by phloem from cotyledons to embryo meristems, where it is used in construction or respired for energy.

FIGURE 11-22 Trees in cold winter weather are not necessarily dormant; sunlight warms them enough for metabolism to occur. Although respiration rates are low, it provides enough energy for critical metabolic reactions. The conifers still have chlorophyll present, so even photosynthesis may be possible.
In thick tubers (potatoes) or bulky roots (beets, carrots), it is not well established whether respiration is completely aerobic or at least partially anaerobic. Such organs usually have a significant amount of intercellular space through which oxygen might diffuse, but the concentration of oxygen is very low. Similarly, sapwood and cambium of trees with thick bark may be hypoxic; even though lenticels are present, the diffusion path is long, and the dense inner bark, cambium, and sapwood lack intercellular spaces. Developing embryos inside large seeds and fruits are reported to respire anaerobically.
Internal Regulation
Like virtually all other processes, respiration is subject to specific metabolic controls. Cells that have an active metabolism, such as glands that secrete protein or epidermal cells that secrete waxes, have a high level of aerobic respiration, whereas meristematic cells produce ribose by means of the pentose phosphate pathway. Nearby cells, perhaps those of the spongy mesophyll or collenchyma, may have a much lower respiration rate (FIGURE 11-23). During fruit maturation, respiration usually remains steady or increases gradually until just before the fruit is mature, at which point a sudden burst of respiration is triggered by endogenous hormones. Conversely, within seeds, after an embryo is mature, its respiration decreases so dramatically that it is difficult to measure, and the seed becomes dormant. In seeds with a true dormant period, virtually no respiration occurs even if the embryo is surgically removed and given water, warmth, and oxygen because metabolic inhibitors suppress respiration. Although chloroplasts produce large amounts of ATP while photosynthesizing, apparently none of that is exported to the cytoplasm: Respiration is the source of ATP for all parts of a cell other than chloroplasts.
![]() Total Energy Yield of Respiration
Total Energy Yield of Respiration
During anaerobic glycolysis, four molecules of ATP are synthesized, whereas either one or two ATPs must be used to initiate the process, depending on whether glucose or glucose-6-phosphate is the initial substrate. The NADH + H+ generated cannot be used for energy, and thus, the net result is two molecules of ATP for every molecule of glucose fermented (TABLE 11-3).
During aerobic respiration, glycolysis again yields two ATPs directly; in addition, the two NADHs can be transported to mitochondria, where their electrons power the formation of two or three more ATPs. The conversion of each pyruvate to acetyl CoA yields another NADH. Because two pyruvates are produced from each initial glucose, six more ATP per glucose are produced. Within the citric acid cycle, each original molecule of glucose yields two molecules of ATP, six of NADH, and two of FADH2; the total is 24 ATPs. Aerobic respiration can produce as many as 38 molecules of ATP, making it significantly more efficient than anaerobic respiration.
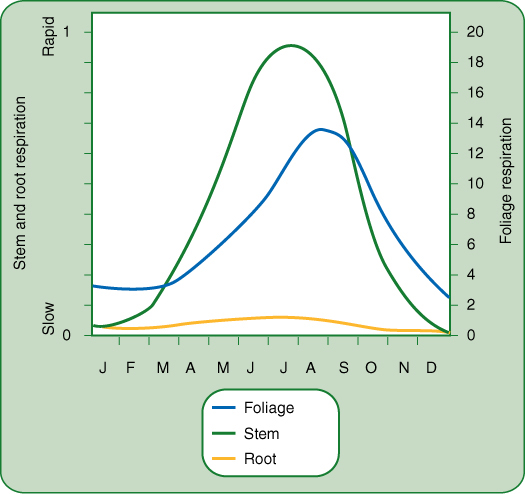
FIGURE 11-23 Respiration rates for various parts of loblolly pine trees in a 14-year-old plantation. Respiration is much higher in summer than in winter for all organs, and leaves have the highest rate—20 times the scale for the other organs. The rate is given as “grams of carbon dioxide released per square meter of soil surface per hour.” This type of measurement is more useful to a forest manager than to a cell physiologist. Different scientists or studies need different types of measurements of the same function. A cell physiologist might express respiration as “grams of carbon dioxide released per cell per hour,” “per gram of protein per hour,” or “per gram of tissue per hour.” Each gives a different result: Leaves have small cells with thin walls, so a gram of leaf tissue has much protein and many cells, whereas a gram of woody stem or branch is mostly inert, nonrespiring cellulose walls with few cells and little proteinaceous protoplasm. It can be difficult to select an appropriate basis for stating rates of respiration, and comparing studies that use different bases is not easy.
TABLE 11-3 Production of ATP by Various Types of Respiration
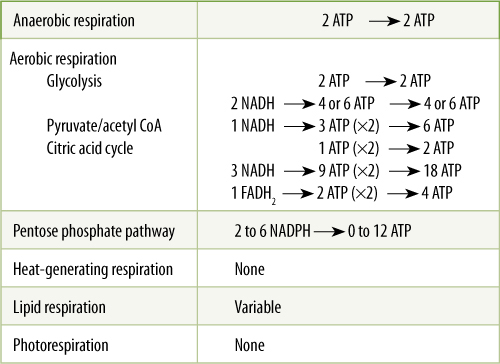
The pentose phosphate pathway yields only two NADPH per glucose-6-phosphate if either ribulose-5-phosphate or erythrose-4-phosphate is drawn off for anabolic metabolism. If neither of these is removed, the various intermediates continue to cycle until all carbon of the glucose is completely oxidized to carbon dioxide and six NADPHs are produced. These may be used for cellular reductions of nitrate to amino acids, sulfates to sulfhydryls, or carbohydrates to fats. NADPH not consumed in anabolic reductions may contribute protons to the mitochondrial electron transport chain and indirectly produce two molecules of ATP.
The amount of ATP produced by fatty acid respiration depends on the length of the fatty acid and whether acetyl CoA enters the citric acid cycle; with maximum respiration, up to 40% of the total energy in a fatty acid is conserved in ATP. Thermogenic respiration and photorespiration produce no ATP. Also, keep in mind that intermediates may be diverted from all respiratory pathways and be used in synthetic reactions, so complete respiration may not occur; ATP production thus is less.
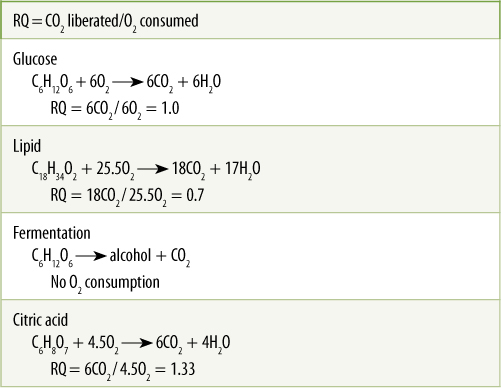
TABLE 11-4 Respiratory Quotients of Various Compounds
![]() Respiratory Quotient
Respiratory Quotient
An action spectrum is a valuable tool for studying light-mediated phenomena such as photosynthesis. For respiration, a similar type of information is useful. A theoretical calculation can be made of the amount of oxygen consumed by each type of respiratory substrate. For example, the complete aerobic respiration of glucose should consume six molecules of oxygen and produce six molecules of carbon dioxide (TABLE 11-4). This ratio of carbon dioxide liberated to oxygen consumed is known as the respiratory quotient (RQ); for glucose RQ = 1.0. Acids can enter the citric acid cycle and be oxidized to carbon dioxide, producing NADH for the mitochondrial electron transport chain. Because acids contain relatively large amounts of oxygen in their molecular structure, less is needed to convert them to carbon dioxide and water, so their RQ is high, greater than 1.0. On the other hand, fatty acids contain virtually no oxygen; therefore, when they are respired, enough oxygen must be consumed to oxidize not only every carbon but every hydrogen as well; thus, the RQ is very low, often about 0.7. Of course, anaerobic respiration consumes no oxygen, although its carbon dioxide production is very high.
After these RQ values are calculated, it is relatively easy to measure the amounts of gases exchanged during respiration and thus gain information about the respiratory metabolism. For example, peanut seeds are rich in lipids and oils. As they germinate, the seedling’s RQ is initially low, indicating that the lipids are being respired. If they are kept in the dark, the RQ remains low until the seedling exhausts its nutrient reserves and dies. But if they are allowed to germinate in the light, the RQ gradually increases toward 1.0 as photosynthesis begins to produce glucose needed for respiration (FIGURE 11-24). The increase is not immediate because much of the first products of photosynthesis are used for constructive anabolic reactions in the leaf cells, so a rise in the RQ indicates that leaf photosynthesis is meeting anabolic needs and producing extra for respiration.
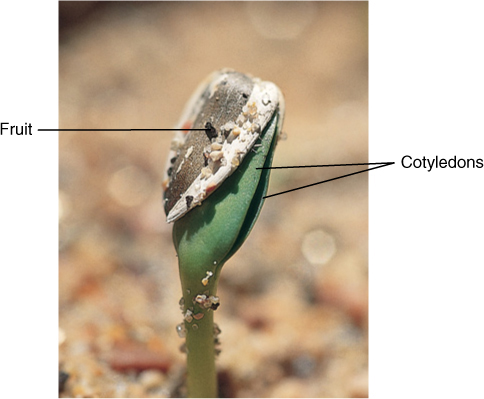
FIGURE 11-24 Sunflower seeds store oils in their cotyledons; when they germinate, the oils are respired, giving the seed a low RQ value. The cotyledons of this seed, although still enclosed by the fruit, have turned green and are carrying out photosynthesis, so both oils and sugars are available for respiration. After a few days, all the oil will be consumed; then only photosynthetically produced sugars will be available, and the seedling will have a high RQ value, near 1.0.
![]() Fermentation of Alcoholic Beverages
Fermentation of Alcoholic Beverages
The ethanol of alcoholic beverages is always produced by the fermentation (anaerobic respiration) of glucose by yeasts. Sugars present in fruits can be fermented immediately, but starches in seeds and tubers must be depolymerized to glucose before they can be fermented. Ethanol produced by fermentation finally kills the yeast if it builds up to a concentration of about 18%. To obtain a stronger concentration of alcohol in the beverage, the fermented mixture must be distilled, a process in which it is heated to concentrate the alcohol while removing some of the water. Alternatively, extra alcohol can be added to a beverage rather than distilling it. These factors combine to give us three basic types of alcoholic beverages: beers, resulting from partially fermenting starchy seeds; wines, produced by a more complete fermentation of sugary fruits; and spirits, in which their ethanol concentration is increased by distillation or by adding alcohol.
Beer
Beer is made by fermenting starchy cereal grains, especially barley, wheat, corn, or rice. Barley is by far the most common type of beer, and if a different grain is used, it is typically specified. For example, beers made from wheat are becoming more popular and are always called “wheat beer.” Before a cereal grain can be fermented, at least part of its starch must be converted to glucose. This is done by moistening the grains and allowing them to germinate. The moistened embryo secretes enzymes that break down the starch located in endosperm. Barley has the greatest number of enzymes and converts starch to sugar more quickly than do other grains. Once the seedling root is visible, sprouting has proceeded long enough, and the grains are dried to prevent any further enzymatic reactions. Sprouted, dried barley grains are called malt (this is also the malt in a malted milkshake). To brew the beer, malt is mixed with water in a large vat; the mixture is called the mash. If malt is the main ingredient, the beer will have a strong flavor, and the quality of the sprouting and roasting of the barley is very important. But often, at least in the United States, unsprouted grains or even corn syrup or potatoes are added. These are very cheap sources of extra starch that can be acted upon by the enzymes in the malt. By adding these adjuncts the beer is less expensive and typically has less flavor.
Hops are the dried carpellate inflorescences of a tall, vining plant, Humulus lupulus, closely related to Cannabis, marijuana. Hops have a bitter taste and aroma and are added so beer will not be too sweet: the greater the amount of hops, the more bitter the beer.
Once all the ingredients are in the mash, it is warmed to 68 to 73°C and allowed to stand for several hours while the various enzymes are active. The chaff and solid materials are then strained out and the sugar-rich liquid, called wort, is boiled to inactivate the enzymes and kill any microbes. Once it has cooled, yeast is added. For beers that will be ales, bitters, or stouts, Saccharomyces cerevisiae is used, but for lagers and pilsners, S. uvarum is added. Most beers in the United States are lagers. The yeasts are allowed to ferment the wort from 1 week to 12 days. The beer is filtered and pasteurized to stop all further fermentation and then is aged for 2 or 3 weeks. The carbon dioxide produced by fermentation is allowed to escape during brewing, so to make beer frothy when it is poured, new carbon dioxide is added to the beer artificially just before it is bottled.
Various types of beer result from controlling the malting process, the adjuncts, hops, and the type of yeast used. S. cerevisiae, the yeast used for ales, floats to the top of the fermenting vats (called top fermentation), and it works best at a warm temperature. This species of yeast produces compounds called esters that add light, fruit-like flavors to the beer. Common types of ale are brown ale, pale ale, and porter (or stout, made with extra hops and more caramelized barley). S. uvarum, used for lager beers, sinks to the bottom of the vats (bottom fermentation) and functions at a cool temperature. Cool, bottom fermentation does not produce esters, so lager beers have a crisper taste. Most of the beer consumed in the United Stated is pale lager, with an alcohol content of only 3% to 6%. The concentration of alcohol depends on the amount of starch converted to fermentable sugar and whether fermentation is artificially stopped at a particular point. With a greater conversion of starch to sugar and a longer fermentation, the concentration of alcohol increases.
Light beers have fewer calories than ordinary beer. This can be achieved in either of two ways. One is to begin brewing with a mash that contains fewer carbohydrates. When brewing is halted, regular beer still has many starches and unfermented sugars present, but light beer has fewer of them. This gives the beer fewer calories. Many people believe that most calories in beer come from the alcohol, which may be true for light beers but not for regular beers, and certainly not for stouts. These have relatively high amounts of starches and sugars, giving the beer its body and fullness. The second way to make light beer is to convert more of its starches to sugars; this ferments to a beer with much higher alcohol content but low in starch. Water is added at the end to lower the alcohol content to the normal 3% to 6% level for beer, but it also dilutes the flavor.
Sake is fermented rice. It is usually called rice wine, but because it is a cereal, technically it should be classified as a beer. However, sake is made by a unique process that differs from brewing beer. The rice is polished to remove all fruit and seed coats and then is steamed to cook and soften its starch grains, as if it were to be eaten. Instead, it is inoculated with the fungus Aspergillus oryzae and allowed to set for several days. Enzymes from the Aspergillus depolymerize the starch to fermentable sugars. After the rice gets a distinctly moldy aroma, it is mixed with warm water, and then S. cerevisiae is added. No hops or adjuncts are added as they would be for beer, and instead of fermenting for only a week, sake is fermented for almost a month. During this time the Aspergillus enzymes continue to convert more starch to sugar, and the yeast ferments that to ethanol. At the end, the sake is separated from the solids by filtration through cloth, and extra ethanol is added to bring the final concentration up to about 20% to 22%; this makes it a fortified beer. Sake is thus much stronger than beer, and it is served hot.
Plants and People
BOX 11-4 Respiration
For the most part people do not affect plant respiration very much, and plant respiration does not have too much of an impact on people. Food storage is one aspect of respiration in which plants and people influence each other. Stored fruits and vegetables respire if they are alive, and if they are moist like fresh potatoes, carrots, peaches, and lettuce they respire especially rapidly. Even dry foods like beans, lentils, wheat, and rice respire, but very slowly. Frozen and canned foods are dead and do not respire.
Respiration in dry foods is important because of the water produced. Remember that the formula for respiration is C6H12O6 + 6O2 → 6CO2 + 6H2O. This water is released from the mitochondria and moistens the cell’s protoplasm, causing the food to become wet and thus susceptible to attack by fungi (molds and mildews). Even if not attacked by fungi, the extra moisture may cause the cells to become more active metabolically, and seeds might even germinate while in storage. To keep wheat, corn, and similar foods dry, air is blown through the storage containers to remove any moisture produced by respiration (FIGURE B11-4A).
In moist stored foods, the extra moisture produced by respiration is not much of a problem. A fresh watermelon has so much water already that the little bit produced by respiration is insignificant. Instead, the problem is that respiration uses up part of their carbohydrates, the very reason we cultivate and harvest most of them. Each week that these plants are stored, there is less carbohydrate available to us when we eat the food. A fresh potato is packed with starch, whereas a potato that has been stored for months has respired away much of its starch and is much less nutritious to us. Respiration in such foods is minimized by keeping them cool. Low temperatures slow down metabolism and thus slow down respiratory loss of carbohydrates.
Sometimes we harm plant respiration indirectly, without even realizing it. We pollute rivers and lakes with fertilizers; this happens when we dump sewage, even cleaned up sewage, into rivers. The wastes we flush down our toilets are food to many types of bacteria, and such bacteria are able to grow rapidly and respire rapidly. With enough human fertilizer, the bacteria proliferate and use up most of the oxygen available in the water or in the mud on the bottom of a lake or river. Plant roots in those areas may then suffer from hypoxia (low oxygen) or anoxia (no oxygen), and if the roots die, the plants may die also. Of course, fish need even more oxygen than roots do, so hypoxia caused by excessive bacterial growth may cause fish to die. A current problem is that the Mississippi River carries huge amounts of human waste, as well as that from farm animals such as cattle, pigs, and chickens. In addition, it also is polluted with agricultural fertilizers that are washed out of fields by rain. As the Mississippi’s water reaches the Gulf of Mexico it fertilizes the bacteria there; the bacteria grow and use so much oxygen that no fish or algae are able to survive. Our pollution causes a huge dead zone in the Gulf of Mexico (FIGURE B11-4B). Similar dead zones occur in other oceans where rivers drain fertilizer-enriched water from farms and cities.

FIGURE B11-4A Grains such as wheat, rice, and corn can be stored in “elevators” like these for months or years, but only if they are kept dry. Grains are never harvested and put into storage unless they are already extremely dry (they must not be harvested during rainy periods). Even so, the very slow respiration of each kernel produces a tiny bit of water, and if it accumulates, fungi can grow and spoil the grain. The elevators have large fans that blow dry air through the grain.
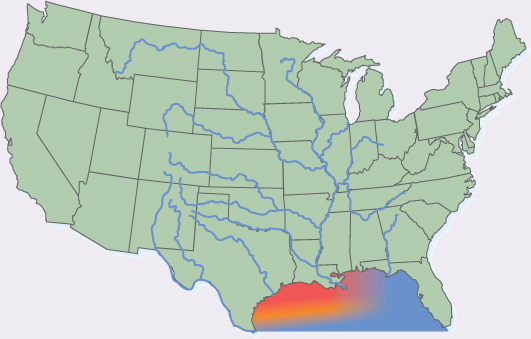
FIGURE B11-4B The dead zone in the Gulf of Mexico is caused by the rapid growth and respiration of bacteria, which are nourished by fertilizers that pollute the Mississippi River and its tributaries.
Wine
Wines are fermented fruit juices that are rich in sugars. When the word “wine” is used by itself, it refers to fermented grapes of the species Vitis vinifera; if other fruits are used, then they are named, such as elderberry wine or peach wine. As anyone who has shopped for or selected wine knows, there seems to be an endless variety of wines, such as Chardonnay, Pinot Noir, Cabernet Sauvignon, and Merlot. It may seem that these represent numerous species of grape, but actually all are varieties of just one, V. vinifera. Wine was first produced about 6000 BCE, and since then farmers have selected numerous varieties (there are now 15,000 varieties), each with its own special flavors, aromas, and colors, but always cultivated from V. vinifera. This is a little surprising because there are 60 species of grape in the genus Vitis. These others are typically used for jams, jellies, juices, raisins, or for eating fresh (table grapes are Vitis labrusca), but they are not used for wine.
Grapes are harvested when they reach proper maturity and their sugar content is suitable. Typically, 20% to 30% of the grape’s weight is sugar (mainly glucose and fructose) when harvested. They are washed and then crushed to obtain the juice. For white wines the skins (exocarps) are removed, but for red or rosé wines the skins are mixed in with the juice. The skins not only provide the color of red wine, they produce various flavors and aromas. The chemical resveratrol is produced when yeasts attack the skins; there are claims that resveratrol lowers blood pressure, prolongs life, and has anticancer activity, but none of these has been proven definitively in humans. S. cerevisiae is added to the juice, and the mixture is cooled slightly; white wines are fermented at 10 to 15°C (50—59°F) and red wines slightly warmer at 25 to 30°C (77—86°F).
Fermentation vats have valves that allow carbon dioxide to escape without allowing oxygen to enter. If oxygen were to enter the tank, certain bacteria would convert the ethanol to acetic acid, which would turn the wine into vinegar. After about 8 to 10 days the liquid is removed from the skins and allowed to continue fermenting for up to 1 month. During fermentation, dead yeast cells and other particles settle to the bottom of the fermentation tank, and crystals of tartaric acid (cream of tartar) also sink to the bottom. The wine must be drawn out of the tank carefully without disturbing the sediment because wine with sediment is less appealing. The tartaric acid crystals are a hallmark of grape fermentation, and archaeologists search from them in old pots to ascertain whether particular peoples were producing wine at a certain time or place.
Fermentation continues as long as sugar is present and the concentration of ethanol has not reached lethal levels (18—20%). If all the sugar is fermented, the wine is a dry wine, but if some sugar remains, it is a sweet wine. Most wines produced in the United States have an alcohol content between 12% and 14% and have some sugar left, so their fermentation must be stopped artificially. This is usually done by microfiltering the wine to remove all yeast; the alternative is to heat the wine enough to kill the yeast (to pasteurize the wine), but that damages the flavor.
At this point the wine begins an aging process. White wines are aged only briefly, between 12 and 18 months, but red wines may be aged for up to 5 years. Typically, wine is aged in large oak barrels, where chemicals in the wine interact with compounds in the heartwood. These reactions alter the flavor and aroma of the wine and even create new types of longer-chain alcohols that give the wine a more viscous, substantial feel in the mouth. When the wine master has decided the wine is ready, it is transferred to bottles and either shipped to market or set aside for more aging in the bottles.
Champagnes and sparkling wines are made by adding sugar to a bottle of wine that still has a few live yeast cells in it. The wine then continues to ferment, but all the carbon dioxide is trapped inside and builds up pressure. When the bottle is uncorked, the carbon dioxide forms bubbles and makes the wine effervesce (or, if not done properly, the wine shoots across the room). To be called “champagne” the wine must be produced in the Champagne region of France. All other effervescent wines are “sparkling wines,” although those of Italy are usually referred to as prosecco. Inexpensive sparkling wines are made by simply carbonating white wine in the same way that soft drinks are carbonated.
If extra ethanol is added to a wine, it becomes a fortified wine. Examples are sherry, port, and Madeira. Such wines are then aged, either in bottles with no exposure to oxygen or in oak barrels that permit a slight oxidation and evaporation, giving the wine a richer feel in the mouth. For example, tawny port is made by stopping fermentation while half the grape sugars are still present. Distilled spirits are added to bring its alcohol level to 20%, and then the wine is aged for 10 years in oak barrels that allow a very small amount of oxygen to act on the mix, converting it from wine to port. During this time the bright red pigment of the grape skins precipitate and change color to a tawny brown.
The effects of global climate change could be devastating for vineyards and wine production. Even with irrigation and a sunny climate, weather still plays a crucial role in the quality of the grapes. Vineyards are typically some of the most costly land used for agriculture, and all the production facilities are located close to most vineyards; juice is rarely shipped more than a few miles. If an area’s climate changes to one slightly warmer or wetter, if the date of the last frost of spring changes, or if rains fall at a different time, wine grapes could be rendered useless. Most existing vineyards have already been carefully chosen to be suitable for a particular variety of grape and for the production of a particular type of wine, so any change in weather or climate will almost certainly hurt the crop rather than improve it.
Spirits
Spirits are alcoholic beverages with an ethanol content above 20%. Once fermentation has produced enough alcohol to raise its concentration to 20%, the solution is toxic to the yeast and they die. To obtain a higher alcohol content, either strong alcohol (always ethanol) must be added or the solution must be distilled. During distillation the solution is heated, which mostly causes the alcohol to evaporate, along with some water and some of the flavoring compounds. Distillation is possible because different liquids boil at different temperatures, and ethanol boils at 78°C (173°F), which is much cooler than the boiling point of water at 100°C (212°F). By keeping the mixture above 173°F and below 212°F, ethanol and certain flavors evaporate and most water remains behind. The vapors are carried away to a pipe that is chilled so the vapors condense into a new liquid, one that has a higher concentration of alcohol, less water, and an altered set of flavorings. This second liquid can be redistilled to raise the alcohol even higher and to achieve a different flavor. Distillation can raise the alcohol content only to 95.6%, making it 191 proof liquor; the “proof” number is twice the alcohol content.
The type of spirit that results from distillation depends on the initial fermented material. Distillation of grain-based fermentations produces whiskeys, vodka, and gin. For example, Scotch and Irish whiskies (spelled with “ie”) use primarily or only malted barley, but American whiskeys (spelled with “ey”) use up to 80% corn as well, and rye and wheat may also be added. Gin also begins with the fermentation of malted barley, corn, and rye, but during distillation juniper berries are added for their flavor and aroma. Vodka is basically just pure ethanol and water: The objective of distillation is to raise the concentration of ethanol and to eliminate all flavors. Consequently, the initial fermentation for vodka is made with whatever carbohydrate is cheapest: wheat, corn, potatoes, and even sugar beets. Brandy results from distillation of grape wine (if fruit wines are distilled, they produce fruit brandies). Distillation of fermented molasses creates rum, whereas tequila and mescal are distilled from fermented juices of agave plants: tequila from Agave tequilana and mescal from A. americana. Both agaves are unusual in that they store their carbohydrates not as starch (a polymer of glucose), as most plants do, but as inulin, a polymer of fructose. The agaves must be cooked to break the inulin down to simple sugars before fermentation can start. Tequila agaves are steamed, whereas those for mescal are roasted slowly (FIGURE 11-25; despite its name, mescal does not contain mescaline).
Warnings
Fermentation of plant material always produces ethyl alcohol (ethanol), and this is classified as a depressant. This seems counterintuitive, because light drinking causes people to be more lively and less inhibited, but even moderate drinking reduces a person’s ability to focus their attention and slows their reaction speed.
Drinking ethanol has both beneficial and harmful consequences. Moderate consumption, about two drinks per day (two bottles of beer or two glasses of wine or two ounces of liquor), lowers the risk of heart disease. Red wine seems to be the most beneficial because it has resveratrol (see above). In contrast, deaths related to ethanol—including driving while under the influence of alcohol (DUI, drunk driving)—is the fifth greatest cause of death among North Americans. Also, drunk drivers don’t just kill themselves; they kill and maim innocent bystanders. Ethanol is not at all addictive to many people, but it is to others and results in alcoholism. Ethanol easily passes from mother to fetus through the placenta and can cause fetal alcohol syndrome if the mother consumes too much ethanol while pregnant. The effect of ethanol on us depends on the amount we drink in proportion to our body size, which in turn determines how much ethanol passes into our blood. When the concentration of ethanol in our blood (our blood alcohol content [BAC]) reaches 0.02%, it starts to interfere with both our coordination and our reaction time, and it may cause us to have impulsive behavior. At a BAC of 0.15% people are drunk and have trouble walking or doing other simple things. A BAC of 0.4% is usually fatal. In the United States it is illegal to drive if your BAC is 0.08% or higher. As a general rule a person can metabolize the ethanol in one standard size drink in about 60 to 90 minutes; if you drink faster than that, chances are good that you will get drunk.

FIGURE 11-25 Mescal is made from Agave americana, which has long, spiny leaves but which stores its carbohydrate in its broad, short stem. The leaves must be cut off, leaving just the stem (the “heart”), which you see here. These hearts are being transferred from the truck to a stone-lined pit; burning charcoal will be added and the entire pit will be covered to allow the hearts to roast slowly, converting their carbohydrates to simple sugars that can be fermented.
Ethanol is only one of many types of alcohol. Other types we encounter commonly are isopropyl alcohol (isopropanol), used as rubbing alcohol, and methyl alcohol (methanol), used as wood alcohol. All alcohols are poisonous, but our bodies are able to detoxify ethanol if we drink only small amounts. With larger amounts we develop a hangover, and even larger amounts cause death. One to several college students die every year from drinking so much ethanol (usually high strength liquor) that it disrupts the lipids in their cell membranes (remember that biologists kill and preserve specimens of plants and animals in a mixture of ethyl alcohol and formaldehyde). Methyl alcohol is very toxic by itself, and our livers convert it into the even more poisonous substances formaldehyde and formic acid. At doses too low to kill us, methyl alcohol causes blindness. Isopropyl alcohol is converted to acetone in our bodies; you may be familiar with acetone as fingernail polish remover.
 At the Next Level
At the Next Level
1. Ecology of respiration. The enzymes involved in respiration respond to temperature: Respiration is more rapid when it is warm, slower when it is cool. Surprisingly, plants do not seem to control respiration very well, and on warm nights, they respire more than they need to, producing unnecessary amounts of ATP that are then wasted. In greenhouses and nurseries, temperatures are kept cool to prevent excess respiration, which is the same reason for refrigerating fruit and vegetables. With global warming, plants will respire more than they do now, and that may affect their fitness. This is a new concern and you may have trouble finding much information, but it will be worth your while.
2. Biology of mitochondria. Mitochondrial biology is more difficult to follow than plastid biology because mitochondria are almost invisible by light microscopy and never contain pigments or granules. But we know that mitochondria divide and multiply as cells divide and grow. In some algae, all mitochondria fuse into just one mitochondrion prior to cell division, then that mitochondrion divides into two when the cell does, and afterward each daughter mitochondrion breaks into numerous smaller mitochondria. There have been fewer projects to sequence the DNA of mitochondria in plants, but we are learning a great deal about the evolution, development, and function of mitochondria and their genomes. Our knowledge of their electron carriers and membranes is much greater than it was just a few years ago.
3. Will higher levels of carbon dioxide in the atmosphere inhibit respiration? At present the concentration of carbon dioxide in the atmosphere is 400 ppm and is expected to reach 700 ppm by the end of this century. The rate of any chemical reaction is influenced by the concentration of the reactants and products, so the increased concentration of carbon dioxide may be expected to inhibit respiration. Experiments in laboratories show little effect, but field experiments, in which the atmospheric concentration of carbon dioxide is altered across a large experimental plot of land, show that plants do respond to elevated carbon dioxide. We are not certain what is happening, because increased carbon dioxide also alters cytosolic acidity, but this is an area you might want to investigate on your own.
SUMMARY
1. Respiration provides a cell with both ATP and small carbon compounds that are important in various metabolic pathways.
2. The type of respiration carried out by a cell depends on environmental factors and the state of differentiation of the cell.
3. Anaerobic respiration—fermentation—is inefficient because only two ATP molecules are produced for each glucose respired; however, under anaerobic conditions, it is selectively more advantageous than its alternative, death.
4. Some plant and animal tissues are facultatively anaerobic, but no large plant or animal can live for prolonged periods without aerobic respiration. They are all obligate aerobes.
5. Three common electron acceptors are oxygen, acetaldehyde (in plants), and pyruvate (in animals); only oxygen is abundant and cheap and results in a harmless waste product, water.
6. During the complete aerobic respiration of glucose, electrons are removed and transported, ultimately, to oxygen. As oxygen accepts electrons, it picks up protons, and water is formed.
7. Aerobic respiration consists of glycolysis, the citric acid cycle, and oxidative phosphorylation via the mitochondrial electron transport chain. Passage of electrons through the electron transport chain creates a chemiosmotic gradient of protons and hydroxyl ions.
8. Some tissues of some plants generate heat by carrying electrons on an alternative set of electron carriers that do not pump protons; no ATP is formed, so all energy of NADH is released as heat.
9. The pentose phosphate pathway is a complex interaction of metabolites that can produce erythrose (for lignin), ribose (for nucleotides), or NADPH (for reducing power).
10. Respiration increases as temperature increases, within the range of approximately 5°C to 25°C. During warm days and nights, respiration is much more rapid than during cool periods.
IMPORTANT TERMS
acetyl CoA
aerobic respiration
anaerobic respiration
citric acid cycle
cyanide-resistant respiration
cytochrome oxidase
dead zone
Embden-Meyerhoff pathway
ethanol (ethyl alcohol)
facultatively aerobic (anaerobic)
fermentation
flavin adenine dinucleotide (FAD)
flavin mononucleotide (FMN)
fumarate
glycolysis
isocitrate
Krebs cycle
lactate (lactic acid)
obligate aerobes (anaerobes)
pentose phosphate pathway
respiration
respiratory quotient (RQ)
strict aerobes
thermogenic respiration
tricarboxylic acid cycle
REVIEW QUESTIONS
1. Most plants store excess energy from photosynthesis. Name some times when recovery of stored energy may occur. Does energy recovery always occur in the same sites where photosynthetic capture occurred?
2. When a storage organ becomes active after dormancy and mobilizes its reserves, the starch is usually converted to sucrose for transport. Why is starch not transported?
3. The oxidation of NADH to NAD+ requires transferring electrons from it onto something else. What are characteristics the ideal recipient would have? What is the recipient actually used? What does it turn into as electrons are added to it?
4. Cellular respiration falls into two categories. What are the two categories? When is each one used?
5. Define obligate aerobe, obligate anaerobe, and facultative aerobe. Name some type of organism that is an example of each.
6. Under what conditions does plant tissue experience lack of oxygen? How is ATP generated from glucose without oxygen?
7. Glycolysis can proceed only if ____________ is available. If it cannot be regenerated from NADH, the organism will starve, despite having glucose available.
8. The reduction of NAD+ to NADH during glycolysis is a problem. Why is this less of a problem if roots absorb nitrates and sulfates or if the plant is synthesizing fatty acids?
9. Anaerobic and aerobic forms of respiration differ in the ultimate electron acceptors for each process. What is the electron acceptor in each? What are the advantages and disadvantages of each?
10. With oxygen present, not only can NAD+ be regenerated, allowing glycolysis to continue, but even more __________ is formed in the regeneration process.
11. During anaerobic respiration in animals, the electron acceptor is pyruvate. What is the acid (or the anion of which acid) that is formed? During hard, fast exercise, your own muscles probably synthesize this acid. What is the sensation you feel?
12. Plants do not make the acid mentioned in Question 11. What do plants make instead during anaerobic respiration? Pyruvate is converted to _________________, and then NADH reacts with that, forming _____________________.
13. Considering the negative aspects of anaerobic respiration, how could natural selection have produced something so inefficient?
14. What are the three basic parts of aerobic respiration? Which steps occur in mitochondria and which in cytosol?
15. Are the initial steps of aerobic and anaerobic respiration similar or dissimilar?
16. For the citric acid cycle to occur, pyruvate must be transported from _____________ where glycolysis occurs, across the _____________________ to the ____________________. It is transported as a two-carbon fragment called acetyl. What is the name of the carrier molecule that transports it?
17. The text mentions that the benefit in the citric acid cycle is the generation of more ATP, yet at only one step is ATP produced. How is the citric acid cycle involved in more ATP production than just that (Hint: what is the fate of NADH and FADH2)?
18. Examine Figure 11-13. Where are the electron carriers of the mitochondrial electron transport chain located—in the matrix, the membrane, or in the crista lumen? As they carry electrons, some of them also deposit protons (H+). Are the protons deposited in the matrix or in the crista lumen?
19. What is a chemiosmotic potential in mitochondria?
20. The flow of protons from the crista lumen to the mitochondrial matrix can be used to synthesize ATP. Describe how this occurs.
21. During glycolysis, is all of the energy of glucose converted to energy in ATP? Why do compost piles become warm?
22. Some plants generate large amounts of heat. Name two examples. How is the generation of heat beneficial to each of these plants? What is the name of heat-generating respiration?
23. The intermediates of all respiratory pathways can be used in _______________ _________________ to make various compounds. It is not ____________________ that they will be completely oxidized to carbon dioxide and water.
24. The pentose phosphate pathway has some intermediates that are pentoses. What is a pentose? Name the one that is shunted into nucleic acid metabolism.
25. The four-carbon sugar erythrose-4-phosphate is the starting material in the synthesis of many compounds. Name two important examples.
26. Consider a meristematic cell preparing for mitosis and a young xylem cell differentiating into a fiber or a tracheary element; each uses the pentose phosphate pathway, but for different products. Explain why.
27. In most tissues, if the temperature is increased by 10°C, how much is the rate of respiration increased?
28. The total energy yield of respiration relates the number of ATP molecules synthesized per glucose molecule respired. What is the yield for anaerobic respiration? For aerobic respiration? For heat-generating respiration?
29. What is the definition of the respiratory quotient (RQ)? The complete aerobic respiration of glucose consumes six molecules of oxygen and produces six of carbon dioxide. What is the RQ for this process? If you measured the RQ of a plant and found that RQ = 0.7, would you suspect that the plant was respiring lipids or citric acid?
Design Credits: Hummingbird: © Tongho58/Moment/Getty; Green plant cells: © ShutterStock, Inc./Nataliya Hora; Purple tulip: © ShutterStock, Inc./Marie C Fields; Dandelion: © ShutterStock, Inc./danielkreissl; Poppy: © ShutterStock, Inc./Saruri; Plant icon: © ShutterStock, Inc./Vector; Digging man icon: © ShutterStock, Inc./Z-art