Botany: An Introduction to Plant Biology - Mauseth, James D. 2017
Plant Physiology and Development
Transport Processes

Chapter Opener Image: These leaves of Camellia are sunburned because long-distance transport of water to them failed. During a prolonged drought, the soil became so dry that roots could not pull water from it and consequently could not supply the leaves with sufficient water. The guard cells of the leaves would have closed, helping to conserve water, but that is similar to when we are dehydrated and stop sweating: Evaporation can no longer keep organs cool. The leaf overheated and tissues died. Fortunately, the buds are well-protected by bud scales and if the drought ends in time, the buds will make new leaves.
OUTLINE
✵ Concepts
✵ Diffusion, Osmosis, and Active Transport
✵ Water Potential
- Cells and Water Movement
✵ Short-Distance Intercellular Transport
- Guard Cells
- Motor Cells
- Transfer Cells
✵ Long-Distance Transport: Phloem
✵ Long-Distance Transport: Xylem
- Properties of Water
- Water Transport Through Xylem
- Control of Water Transport by Guard Cells
Box 12-1 Botany and Beyond: Water and Ecology
Box 12-2 Plants and People: Farming “Wastelands”
Box 12-3 Alternatives: Desert Plant Biology
LEARNING OBJECTIVES
After reading this chapter, students will be able to:
✵ Define diffusion, osmosis, and active transport.
✵ Recall the three types of membranes.
✵ Explain the components of the water potential equation.
✵ State three important points regarding movement of water.
✵ Describe the changes in osmotic potential and pressure potential when a cell is placed in a water solution.
✵ Restate the importance of incipient plasmolysis.
✵ Summarize the relationship between guard cells, adjacent cells, potassium, and water potential.
✵ Explain the pressure flow hypothesis.
✵ Discuss the cohesion—tension hypothesis.
✵ Describe differences in water availability in various environments.
 Did You Know?
Did You Know?
✵ Air is usually so dry it can pull water out of plants, the same way it makes our skin dry and lips chapped.
✵ Water is pulled upward through plants, similar to lifting an entire icicle simply by pulling up on its top.
✵ In contrast, sugary syrup is pushed through phloem the same way that sweat is pushed through sweat glands.
✵ In late winter and early spring, maple trees conduct sugar through xylem rather than through phloem; maple syrup is concentrated xylem sap.
![]() Concepts
Concepts
One fundamental aspect of life itself is the ability to transport specific substances to particular sites, moving molecules against the direction in which they would diffuse if left alone. After death occurs, atoms, ions, and molecules diffuse, moving from regions of higher to lower concentration, and the organization of protoplasm decays; the disorder of the components increases. Diffusion also occurs during life but proceeds more slowly than the controlled and oriented transport processes that tend to increase the order within the plant or animal body. Transport processes consume energy, and many are driven by the exergonic breaking of ATP’s high-energy phosphate-bonding orbitals.
Specific transport occurs at virtually every level of biological organization: Enzymes transport electrons, protons, and acetyl groups. Membranes transport material across themselves. Cells transport material into and out of themselves as well as circulate it within the protoplasm. Entire organisms transport water, carbohydrates, minerals, and other nutrients from one organ to another—between roots, leaves, flowers, and fruits.
Plants have only a few basic types of transport processes, and the fundamental principles are easy to understand. They are grouped here into short-distance transport, which involves distances of a few cell diameters or less, and long-distance transport between cells that are not close neighbors.
Many types of short-distance transport involve transfer of basic nutrients from cells with access to the nutrients to cells that need them but are not in direct contact with them. Such transport requirements arose when early organisms evolved such that they had interior cells that were not in contact with the environment. Short-distance transport became necessary to the survival of internal cells.
Long-distance transport is not absolutely essential in the construction of a large plant. Many large algae have no long-distance transport, nor do sponges, corals, or similar animals; however, the ability to conduct over long distances is definitely adaptive, especially for land plants (FIGURE 12-1). Before xylem and phloem evolved, a plant’s absorbing cells could not penetrate deep into soil because they would starve so far from photosynthetic cells, nor could they have transported their absorbed nutrients very far upward. Being limited to the uppermost millimeter or two of soil meant that absorptive cells could not reach more permanently moist, deep soil where there are more minerals; the uppermost layers dry quickly and free minerals are leached away by rain. With xylem and phloem, roots that penetrate deeply can be kept alive and their gathered nutrients can be carried up to the shoot.
Vascular tissues make it selectively advantageous for shoots to grow upright, elevating leaves into the sunlight above competing plants. This elevation is feasible because photosynthetically produced sugars can be transported downward to other plant parts. Such elevation of photosynthetic tissues resulted in tall plants that could also place their reproductive tissues at a high elevation, enabling spores or pollen to be distributed widely by wind. After insect-mediated pollination evolved, it was adaptive to have flowers located high, in an easily visible position. The evolution of transport processes affected all aspects of plant biology and permitted later evolution of many new types of plant organization.
Vascular tissues also act as a mechanism by which nutrients are channeled to specific sites, resulting in rapid growth and development of those sites (FIGURE 12-2). At certain times, nutrients are transported to apical meristems, promoting growth and leaf primordium initiation; at other times, nutrients are directed to flower buds or young fruits, and at other times, production of wood and bark is supported. The combination of short- and long-distance transport has resulted in the ability of some plants to become large and complex enough to survive temporary adverse conditions, such as drought, heat, or attack by pathogens.
Because almost everything transported by a plant (or animal) is dissolved in water, the ability of water to move throughout a plant is important. Water is an unusual liquid: It is heavy and viscous, and it adheres to cell components as well as to soil, factors that affect transport processes.
Related to transport processes are isolation mechanisms that inhibit movement of substances. Plants are adept at synthesizing organic polymers impermeable to a variety of substances. The epidermis with its cutin-lined walls keeps water in the shoot after it has been transported there by the xylem (FIGURE 12-3). The Casparian strips of the endodermis prevent diffusion of minerals from one part of a root to another. Isolation mechanisms are essential if transport is to be useful.
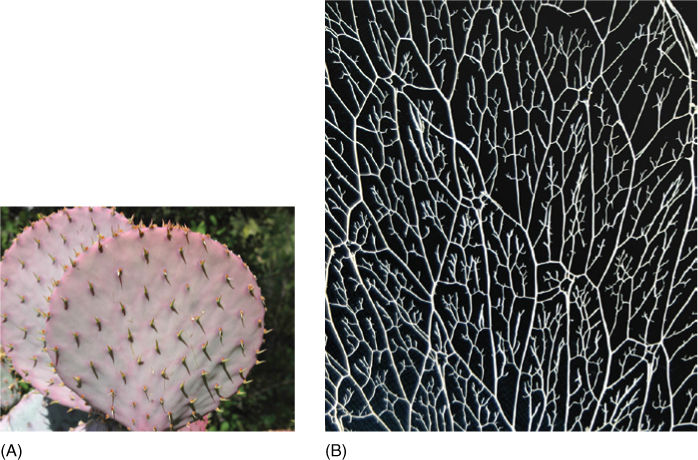
FIGURE 12-1 (A) This is an Opuntia cactus with flattened stems and tiny leaves that will soon abscise. Xylem and phloem are arranged in a net-like pattern, with leaves located at every vertex of the net. Xylem is a means of long-distance transport, carrying water from the tips of this plant’s long roots, up into and throughout this stem. Short-distance transport occurs as water moves out ofxylem and then from cell to cell until it reaches the epidermis. (B) This is the xylem of an Opuntia cactus, as in (A). The soft parenchyma of its cortex and pith rotted away, then the epidermis fell off, leaving only the lignified vascular bundles.
![]() Diffusion, Osmosis, and Active Transport
Diffusion, Osmosis, and Active Transport
The first thing to consider is the mechanism by which material moves through a solution and crosses a membrane. The simplest method is diffusion, in which the random movement of particles in solution causes them to move from areas where they are in relatively high concentration to areas where they are in relatively low concentration. Diffusion through a membrane is technically known as osmosis.
Membranes are of three types: Freely permeable membranes allow all solutes to diffuse through them and have little biological significance. Completely impermeable membranes do not allow anything to pass through and occur as isolation barriers. Differentially or selectively permeable membranes allow only certain substances to pass through; all lipid/protein cell membranes are differentially permeable. Hydrophobic molecules diffuse easily through any cell membrane, whereas many polar, hydrophilic molecules can cross differentially permeable membranes only if the membranes have special protein channels through which the molecules can diffuse. Water molecules, even though highly polar, pass through all membranes, but their movement is more rapid if the membrane has protein channels called aquaporins.
Most membranes also have membrane-bound molecular pumps that use the energy of ATP to force molecules across the membrane, even if that type of molecule is extremely concentrated on the receiving side; this is active transport. The molecular pump, which is a protein, binds to both the molecule and ATP; when ATP splits into ADP and phosphate, the energy is transferred to the pump, forcing it to change shape, carry the molecule across the membrane, and release it. The membrane must otherwise be extremely impermeable to the molecule or it would leak back. Proton pumping in photosynthesis and respiration are examples of active transport.
All cell membranes are important in transport processes; the plasma membrane governs movement of material into and out of the cell. Substances can move across the vacuolar membrane by either osmosis or active transport; the vacuole acts as an accumulation space for sugars, pigments, crystals, and many other compounds (FIGURE 12-4). The endoplasmic reticulum and dictyosome membranes transport material that then accumulates in vesicles. These vesicles may be relatively permanent, remaining in the cell for long periods of time, or they may be a means of intracellular transport, in which the vesicles migrate through the cytoplasm and fuse with another organelle. During fusion, the membranes merge and the vesicle contents are transferred into the organelle. This is a common means of moving material from the endoplasmic reticulum to dictyosomes or from either of these organelles to the cell exterior by fusion with the plasma membrane. During cell division, the new cell plate (the two primary walls and middle lamella) is formed by the coalescence of vesicles from both endoplasmic reticulum and dictyosomes.
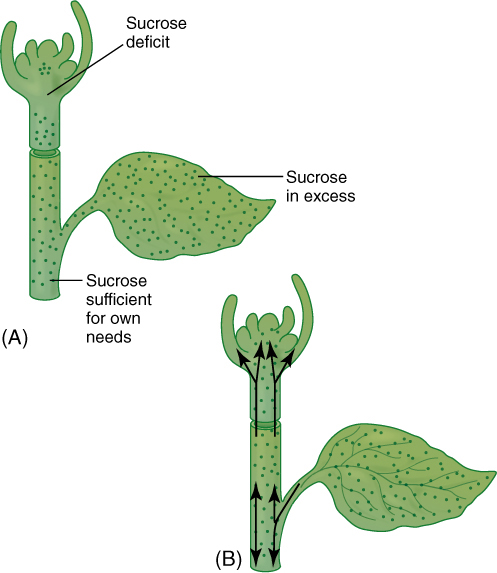
FIGURE 12-2 (A) Imagine an angiosperm that has no vascular tissue. Leaves would produce large amounts of glucose, but because diffusion is slow over long distances, the sugar would diffuse out of the leaf only slowly and in small quantities. The shoot apex has almost no chlorophyll; if it had to depend solely on its own photosynthesis, growth, leaf initiation, and leaf expansion would be extremely slow. (B) With vasculature, glucose can be transported from regions of excess to regions of need; apical meristems and leaf primordia can thereby grow very rapidly. Vascular tissues also make the minerals gathered by an extensive root system available to regions that need them.
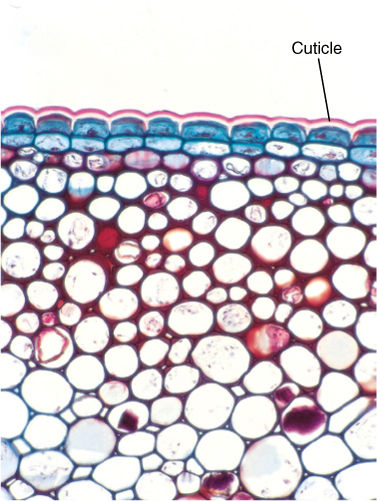
FIGURE 12-3 The cuticle (pink), composed of cutin, is a waterproof epidermal layer that acts as an isolation mechanism, retaining water within the plant and keeping pathogens out (×80).
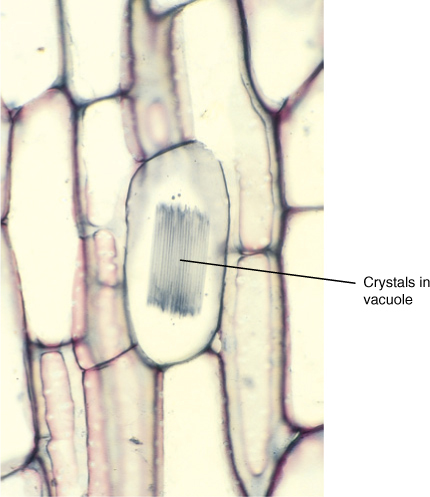
FIGURE 12-4 These crystals are located in a cell vacuole. Calcium and oxalic acid were transported across the vacuole membrane by molecular pumps, concentrating them until they crystallized into these needle-shaped raphide crystals (×80).
![]() Water Potential
Water Potential
Like any other chemical, water has free energy, a capacity to do work. For most chemicals, this energy is called its chemical potential. Because water is so important in botany, its chemical potential is usually referred to as water potential and has the symbol ψ (pronounced “sigh”). Water potential, the free energy of water, can be increased several ways: Water can be heated, put under pressure, or elevated. The energy of water can be decreased by cooling it, reducing pressure on it, or lowering it.
Water’s capacity to do work can be changed in other ways as well. When water adheres to a substance, these water molecules form hydrogen bonds to the material and are not as free to diffuse as are other water molecules; their capacity to do work has decreased. Consider a small beaker of water; if it is pure water, it can flow, move, dissolve material, and hydrate substances, but if a sponge is added, water molecules adhere to the sponge material and can no longer flow or easily dissolve things. If a large amount of sugar is added instead of a sponge, the results are the same. Syrup is just a sugar solution, but the water molecules in syrup have less capacity to do work than do the molecules of pure water.
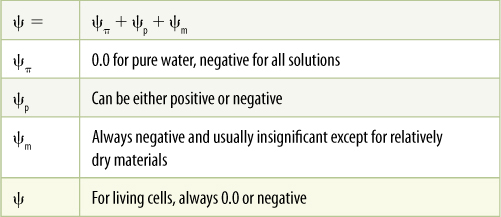
Water potential has three components:
ψ = ψπ + ψp + ψm
In this equation, ψp is pressure potential, the effect that pressure has on water potential. If water is under pressure, the pressure potential increases and so does water potential. If pressure decreases, so do the pressure potential and water potential. Pressure can be positive (when something is compressed) or negative (when something is stretched). Most liquids cannot be stretched very much, but because water is cohesive, it actually can resist considerable tension. When water is under tension, pressure potential is a negative number. Potential is measured in units of pressure, usually in megapascals (MPa) or bars. One megapascal is approximately equal to 10 bars or 10 atmospheres of pressure. Pure water at one atmosphere of pressure is defined as having a water potential of zero.
ψπ (“sigh pie”) is osmotic potential, the effect that solutes have on water potential. In pure water, no solutes are present and osmotic potential is given the value of 0.0 MPa. Adding solutes can only decrease water’s free energy because water molecules interact with solute molecules and cannot diffuse easily; therefore, osmotic potential is always negative. If water molecules do not interact with the added molecules, the substance does not dissolve.
It is important to be careful here: Adding acid to water only seems to make water more active. The solution may have more free energy than the water, but it does not have more free energy than both pure water and the original concentrated acid.
Osmotic potential is related to the number of particles present in solution; that is, a solution composed of 2 g of glucose in 100 mL of water has an osmotic potential twice as negative as a solution of only 1 g of glucose per 100 mL of water. This has some unexpected results: If a molecule of starch containing 1,000 glucose units is hydrolyzed to 1,000 free glucose molecules, the osmotic potential of the solution becomes much more negative because there are now 999 more particles in solution than previously. Using terms such as “increase,” “decrease,” “larger,” and “smaller” can be confusing when dealing with negative numbers. If the osmotic potential goes from —0.01 to —0.1 MPa, is it increasing or decreasing? It is least confusing to use the terms “more negative,” “less positive,” and so on.
ψm is matric potential, water’s adhesion to nondissolved structures such as cell walls, membranes, and soil particles. Adhesion can only decrease water’s free energy, and thus, matric potential is always negative. In soils, matric potential is important because so much of the soil water is tightly bound to soil particles, but in living cells, matric potential usually is much less important than osmotic potential or pressure potential and usually is ignored entirely. The water potential equation for living cells is usually considered to be just ψ = ψp + ψp (TABLE 12-1).
TABLE 12-1 Possible Values of Water Potential Components
Movement of water is related to water potential; substances diffuse from regions where they are more concentrated to regions where they are more dilute. This can be stated more precisely for water: Water moves from regions where water potential is relatively positive to regions where water potential is relatively more negative (FIGURE 12-5). This statement contains several important points:
1. Water moves whenever there is a difference in water potential within the mass of water. All protoplasts are interconnected and most cell walls are fully hydrated; therefore, basically all of a plant body is one mass of water; water can move between regions in the plant if the water potentials of the regions are not equal (FIGURE 12-6A). As a consequence, the water potential of any particular cell may change many times a day as various parts of a plant lose or gain moisture (FIGURE 12-7).
2. If the water potentials of two regions are equal, the regions are in equilibrium, and there is no net movement of water. Water still diffuses back and forth, but on average, equal numbers of water molecules diffuse into and out of a site (FIGURE 12-6B). Unless frozen, water is always in motion, always moving within a plant from areas where it is abundant or under pressure to areas where it is rare or under tension.
3. Water potentials must always be considered in pairs or groups. Because water moves from one site to another, the water potentials of the two sites are important. Knowing one single water potential does not allow us to predict whether water will move (FIGURE 12-6C).
Temperature is not a factor because the solutions being compared are assumed to be at the same temperature. This is not strictly correct if the water potentials of leaves and roots are compared, but the difference is not significant.
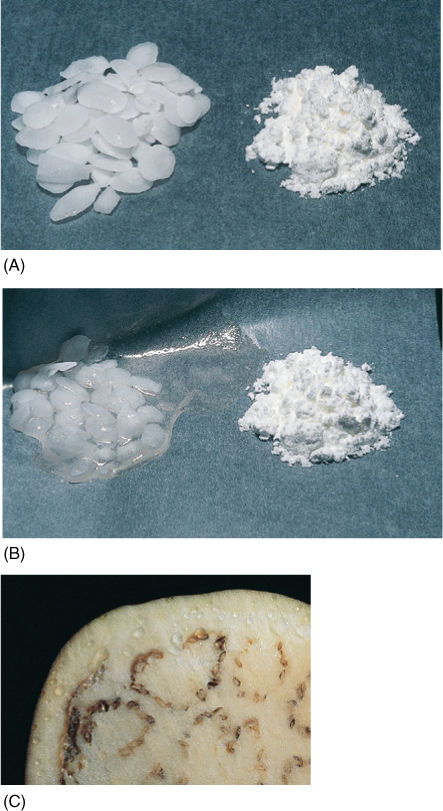
FIGURE 12-5 (A) The material on the left is potassium hydroxide; on the right is starch. They were photographed immediately after being exposed to air, while they were dry. (B) Photographed after 1 hour in humid air. Water has moved from where it was more concentrated (the air) to less concentrated. The potassium hydroxide holds water by forming a solution with a very negative osmotic potential. Water is held to the starch by adhering to the long polysaccharide molecules. Water is not obvious in the starch, but think of saltine crackers left unwrapped. (C) By adding salt to eggplant, water can be drawn from the tissues, making them easier to cook.
Cells and Water Movement
Some examples may help. Imagine a cell with a water potential of —0.1 MPa. It contains solutes that cause the osmotic potential to be some unknown negative number. The cell is turgid and presses against the cell wall, but the cell wall presses back equally, causing pressure on the cell, and the pressure potential is some unknown positive number. We can ignore matric potential because it is usually such a small number. The osmotic potential, whatever it is, plus the pressure potential, whatever it is, equals —0.1 MPa (FIGURE 12-8A). Now imagine the cell being placed in a beaker of solution that also has a water potential of —0.1 MPa. The two water potentials are equal, the cell and solution are in equilibrium, and no net water movement occurs—the cell neither shrinks nor swells. Water molecules do move between the cell and the solution, but approximately equal numbers move in each direction every second (FIGURE 12-8B).
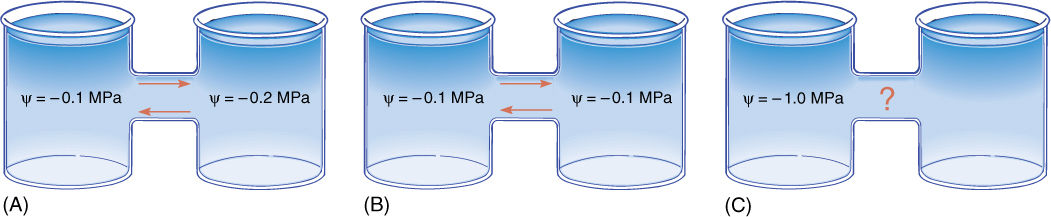
FIGURE 12-6 (A) If a solution whose water potential is —0.1 MPa is connected to a solution whose water potential is —0.2 MPa, the two are not in equilibrium. Water moves from a region of relatively positive water potential to a region of more negative potential, as indicated. Water molecules move in both directions, but more move to the right in any particular instant. Both beakers must have solute dissolved in them (otherwise each would have c = 0.0 MPa), and the —0.2 MPa solution must have twice as much solute. Because the concentration of solute molecules in the left beaker is lower, they are less able to restrict the movement of water molecules than those in the right beaker. (B) If the water potential of two solutions is identical, they are in equilibrium, and no net movement of water occurs; in each second, equal numbers of water molecules move to the right and left. (C) A c of —1.0 MPa is very negative and has a strong tendency to absorb water, but we cannot be certain that water will move to it in this case; the right beaker may contain a solution with a c of —1.1 MPa.
Now imagine the same cell placed in a solution with a water potential of —0.3 MPa (FIGURE 12-8C). The water potentials are not equal, that of the solution being more negative (has more solutes) than that of the cell, and thus, water moves from the cell into the solution. How much water moves? As water leaves the cell, the solutes that remain in the protoplasm become more concentrated. Because more solutes are present per unit water, osmotic potential becomes more negative. As water moves out, the protoplasm volume becomes smaller, so the protoplast presses against the wall with less force and the wall presses back less; therefore, pressure potential becomes less positive. Because both osmotic potential and pressure potential are decreasing (becoming more negative), so is the water potential of the cell. At some point, the cell’s water potential (ψcell) reaches —0.3 MPa and is in equilibrium with the solution; then net water movement ceases (FIGURE 12-8D). Of course, as water moves from the cell into the experimental solution in the beaker, the solution becomes more dilute, and its osmotic potential and water potential become less negative; its pressure potential does not change because pressure cannot build up in an open beaker. Therefore, equilibrium actually occurs slightly above —0.3 MPa, but because most beakers are much larger than most cells, the amount of water that moves is much more significant to the cell than to the beaker solution.
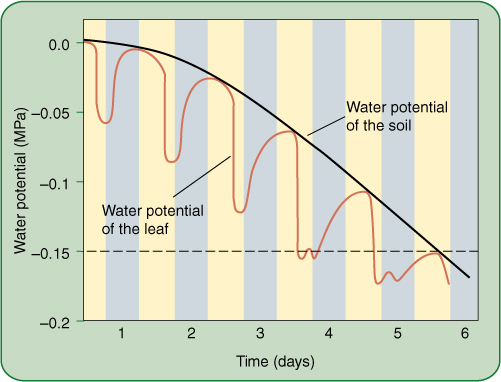
FIGURE 12-7 If soil is watered well and then allowed to dry over a period of days, the water potential of the soil solution gradually and smoothly becomes more negative. Every day (yellow bars), leaves lose water more rapidly than xylem replaces it; therefore, leaves dry slightly, and leaf water potential becomes more negative. At night (dark bars), stomata close and xylem transport rehydrates the leaf tissue, but in each cycle, leaves dry more than the previous night. This is not very serious until daytime leaf water potential becomes more negative than the wilting point (dashed line). When leaves wilt, many metabolic processes are adversely affected. Wilting point varies from species to species and is much more negative for xerophytes than for mesic plants. After soil becomes extremely dry, even nighttime rehydration does not bring leaf water potential above the wilting point; the plant is at its permanent wilting point, and severe stress damage may occur. All growth stops, and leaves and developing flower buds or fruits may die and be abscised.
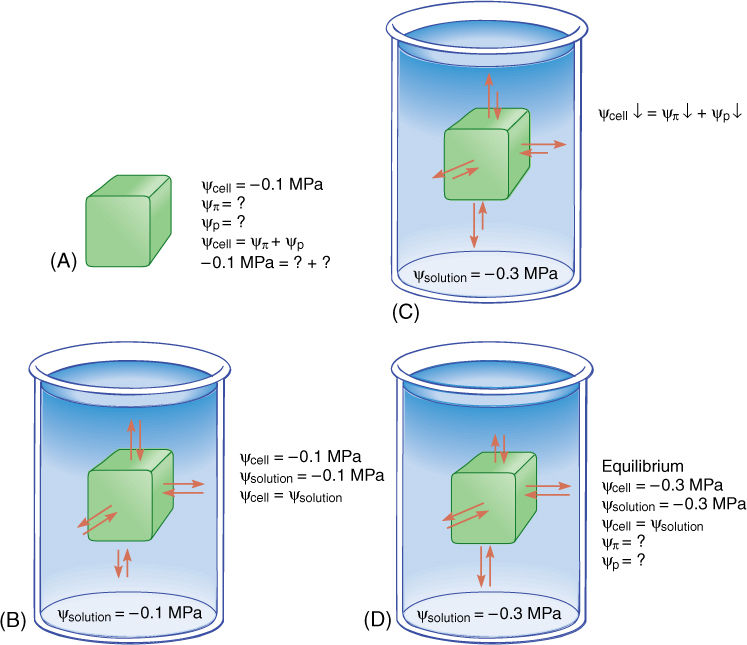
FIGURE 12-8 (A) A cell whose c is —0.1 MPa but whose values of ψπ or ψπ are unknown. (B) If the cell is placed in a solution with a c of —0.1 MPa, the cell is in equilibrium with the solution and no net movement of water occurs. (C) If the cell is placed in a solution with a c of —0.3 MPa, the cell loses water to the solution until the cell’s water potential is also —0.3 MPa (D), even if the cell is killed by loss of water. The fact that the cell’s water potential becomes more negative means that the osmotic potential or the pressure potential or both also become more negative.
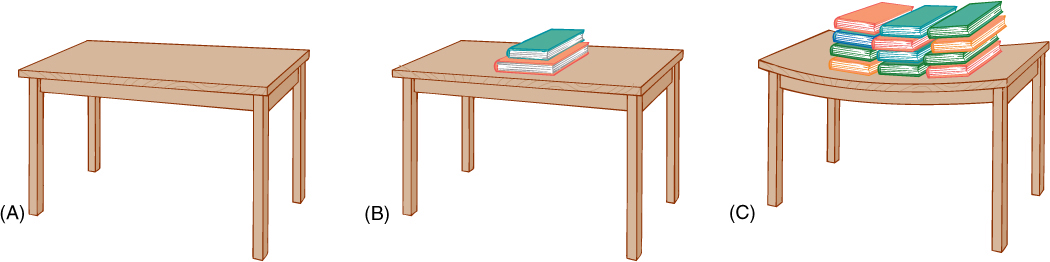
FIGURE 12-9 (A) The molecules in this table top are exerting just enough pressure upward to counteract gravitational force: There is no movement upward or downward of the table top, and thus, forces must be in equilibrium. (B) Adding several books increases gravitational force, but no net movement occurs. The table top is slightly bent, which stretches its molecules, but they resist just enough to counter the new force. (C) Even more force is perfectly balanced by the table top. If the books were removed, the stretching on the table would stop, and the molecules would go back to exerting only enough pressure to counteract its own weight—the table would not fly upward.
Consider the relative importance of osmotic potential and pressure potential in this example. In order for osmotic potential to become twice as negative (e.g., from —0.15 to —0.3 MPa), the cell has to lose half its water or double the number of solute particles. Either action is drastic; almost any cell dies if it loses half its water, and thus, osmotic potential does not usually increase or decrease more than a few megapascals. Pressure potential can change enormously, however, usually with movements of only small amounts of water. Consider the top of a table: Its molecules are pressing upward with exactly the same force that gravity is pulling them downward. Placing a book on the table causes the table’s molecules to exert more pressure upward as their bonding orbitals are stretched (FIGURE 12-9). The table changes the amount of upward pressure it can exert with very little change in shape.
A similar process occurs in cell walls; imagine placing the cell, now with a water potential of —0.3 MPa, in a beaker of pure water. Some water moves inward, diluting the solutes and causing the osmotic potential to become less negative, but the change is not significant; however, the extra volume of the water causes the protoplast to swell and press against the wall with more force. The wall presses back with equal force and pressure potential rises rapidly, even though only a small amount of water moves in (FIGURE 12-10). How high will it rise? We cannot predict its value; however, it will go high enough that osmotic potential plus pressure potential will equal zero, and the cell will be in equilibrium with pure water.
Can a cell ever absorb so much water that it bursts? Animal cells often burst if placed in pure water, a process called lysis, but plant cells can never burst (FIGURE 12-11). Walls, either primary or secondary, are always strong enough to resist breakage by water absorption. Even the thinnest, most delicate walls of mature parenchyma cells can exert enough pressure on the protoplast to raise the pressure potential high enough to counterbalance osmotic potential, however negative it might be.
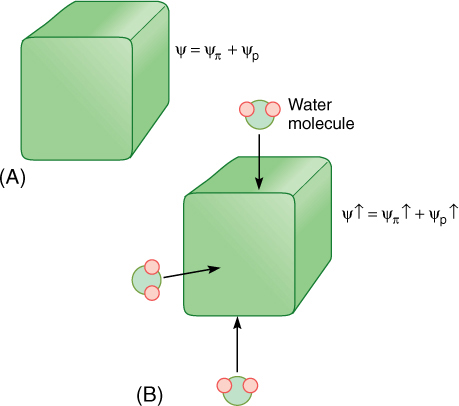
FIGURE 12-10 (A) A healthy cell, turgid and full of water and protoplasm. It is swollen and firm, just like the cells of unwilted leaves. Its walls are stretched and are pressing back against the protoplasm that is pressing on them. (B) If even a small amount of water enters the cell, the slight increase in volume causes the walls to stretch and push back, and pressure builds inside the cell. Consequently, ψp becomes much more positive and so does the cell’s water potential. Because of the slight increase in the amount of water in the cell, the salts, sugars, amino acids, and all other solutes are now slightly more dilute; therefore, ψπ becomes slightly less negative also, but this change is insignificant compared with the change in ψπ.
Immature, growing cells have weak, deformable walls and cannot generate enough pressure to stop water absorption. The cell grows rather than bursts. Under these conditions, the cell may increase greatly in size. With such a large influx of water, solutes in the cell may become significantly diluted; osmotic potential and water potential may become less negative, and the cell reaches hydraulic equilibrium with surrounding cells and growth stops. In growing regions, however, such as the tips of stems and roots, and in expanding leaves, cells can keep their osmotic potential and water potential very negative despite the influx of water, either by actively pumping in solutes through the plasma membrane or by hydrolyzing giant starch molecules into thousands of glucose molecules. After the proper size is reached, growth can be stopped either by strengthening the wall so that it exerts more pressure and raises the pressure potential or by stopping the import of solutes or the hydrolysis of starch, allowing the osmotic potential to rise. Either way, rising pressure potential or osmotic potential causes the cell’s water potential to rise and reach equilibrium with the surrounding cells, stopping the net inflow of water.
Botany and Beyond
BOX 12-1 Water and Ecology
Water is essential to life. The regions of the world that have no water have no life, whereas those areas that do have water—even just a little water—have at least a few organisms. Think about the various ways that water is available to us living creatures; think about aspects of the world that we already know. Use our two analytical questions: What are the alternative ways in which water is present? What are the consequences of each alternative?
![]() The Water Available in Water
The Water Available in Water
Most of the world is covered with water. Oceans, lakes, marshes, ponds, rivers, snow fields, and glaciers occupy more of Earth’s surface than does land. It might seem that there is plenty of water in water, but we know that water comes in a variety of forms—fresh, salty, brackish, and so on. Lakes and rivers contain fresh water, that is, water that has very few solutes dissolved in it. It starts out as rain, water that condenses from clouds and is basically distilled water, pure water. As it collects in streams, lakes, and rivers, this “water” continues to be more or less pure. Mountain streams are clear and clean because the water is too pure for algae to grow in it. Larger rivers are less pure because they have received runoff from fertilized fields, lawns, and golf courses, and cities dump their sewage into rivers. Sewage is always rich in dissolved minerals and organic chemicals, and river water often reaches a balance of water and minerals that supports abundant life. Algae grow so abundantly that they degrade the river. As the algae die, their bodies sink and are decomposed by bacteria, a processes that uses up so much oxygen that fish may suffocate. This process is called eutrophication, but you are probably fortunate enough to have never seen a eutrophied river. In the 1960s, we realized that we could never keep all of our sewage and agricultural runoff out of rivers, but if we could at least keep phosphate levels low, we could prevent eutrophication. Algae can not grow without phosphate. Most phosphate pollution was due to laundry detergents, and since the 1960s, we have switched to alternative detergents that are free of phosphates.

FIGURE B12-1A This small part of Yosemite National Park has many microhabitats that differ in water availability. The waterfall has abundant water when the stream is flowing, but it goes dry or freezes at times. Its spray zone provides some water, but the cliff face is wet only during a rain—it has no moisture holding capacity. Trees grow where soil retains moisture longer than on the cliff face, but some areas with soil are in full sun, whereas others are in shade; thus, rates of transpirational water loss differ.
Oceans contain saltwater. Rivers bring in water and dilute salts, but the water will evaporate, form clouds, and then fall as rain again and run to the oceans, carrying another load of dilute salts. This is a mechanism that moves salts from land to ocean and concentrates them. Currently, the salts in seawater are so concentrated that land plants and animals cannot use ocean water to hydrate themselves: We would die if we tried to survive by drinking seawater just as plants would die if we irrigated them with it. Marine organisms have plasma membranes and vacuolar membranes able to move salts either into or out of the protoplasm as necessary to keep the cells properly hydrated. Seagulls have glands that purify seawater and throw away the salt, but we land organisms cannot do that.

FIGURE B12-1B This cactus Armatocereus procerus grows in a remarkably dry area of coastal Peru. It is shriveled and sunburned from lack of water. It appears that this area receives rain only during El Niño years, and those occur only about once every 10 or 15 years. These plants must routinely go for 10 years or more with no rainfall whatsoever.
Seawater’s ratio of salt to water changes because of many factors. As rivers flow into an ocean, they deposit buoyant fresh water, which floats like a cap on the seawater (salts in seawater make it denser than fresh water). Marine algae cannot live in the cap of fresh water, and must remain in the deeper saltwater, where they do not have as much light for photosynthesis. As the fresh river water spreads out, the thickness of its layer diminishes, and at some point, wave action mixes fresh and saltwater together. The mouth of a river is a complex gradient of fresh and saltwater.
The complex mixing at the mouth of a river is a rich environment that provides numerous habitats for many types of creatures. Unfortunately, we have interfered greatly with these ecosystems. The simplest to understand are the Rio Grande as it flows into the Gulf of Mexico and the Colorado River as it flows into the Sea of Cortez: Both the United States and Mexico use so much of the river water for drinking, manufacturing, and irrigation that these two mighty rivers are nothing more than trickles that barely reach the coast. They no longer provide the millions of gallons of fresh water that would have maintained the coastal ecosystems, and without this outward flow of fresh water, the salty seawater approaches into the mouth of the rivers. Fresh water marshes have been destroyed by brackish water that floods in at high tide.
The concentrations of salt and water in seawater also change greatly at the North and South Poles. The Arctic Ocean near the North Pole is frozen over with a sheet of ice, and at the South Pole, the continent of Antarctica is covered with snow fields and glaciers. Ice and snow are pure water, and as they melt in the summer, they dilute the salts in seawater. It may seem like these are such cold places that they must be lifeless except for a few polar bears in the north and penguins in the south, but that is not true. Polar water is so teeming with microscopic algae and animals that it is one of the most life-filled habitats in the entire world. The addition of fresh water as glaciers and icebergs melt in summer is a problem they have adapted to. In the winter, there is the opposite problem: As water freezes into ice, it leaves its salts behind; therefore, as the poles become more ice bound in winter, the remaining water becomes ever more salty, another dilemma for the creatures in it. An even worse problem is that the increasing saltiness causes increasing density, and the cold, salty water sinks. Fish and seals easily swim upward and remain safe, but algae and microscopic animals are carried downward into darkness where they become food for benthic creatures, those that live on the ocean floor.
The intertidal zone—the region of coast that lies between the levels of low and high tides—is an area where available water changes greatly and quickly. As the tide goes out, sea anemones, barnacles, and algae are at first wet with seawater, but as the water on their surface evaporates, the concentration of salt increases. If exposed long enough, they may become not only dry but encrusted with salt, and they must retain water in their bodies against the osmotically dry salt on them. In contrast, a rain shower drenches them in fresh water, and they then struggle to prevent a loss of minerals out of their bodies.
![]() The Water Available in Air
The Water Available in Air
Air supplies water to land plants in the form of rain, fog, dew, frost, snow, hail, and even just humidity. Air also pulls water out of plants, it is the motive force for transpiration. Water availability is more complex than just the amount of rain that falls per year per square mile. Also important are the timing and regularity of precipitation, as well as the rate at which water is lost between rains. Some desert regions, for example, the Atacama Desert in Peru and Chile, are believed to have never received any rain at all, ever. At the other end of the scale are fog and cloud forests, regions where air is saturated most of the time: Plants are dripping wet and soil is always moist. Far from being ideal habitats, fog forests are populated by dwarf trees, stunted because the humidity prevents transpiration and transport of minerals up from the roots. Most land habitats are more ordinary, having rainy days that alternate with sunny ones, but there are thousands of alternatives here—the ratio of rainy days to sunny ones, the amount of rain that falls each time, the seasons when it falls, the dryness of the air between rains, and so on. Who would guess that Seattle and Austin receive virtually identical amounts of rain? Each get about 35 inches of rain per year, but in Seattle, a bit falls every week, whereas Austin gets 0.6 to 6 inches every other month or so. Between rains, Seattle is cool and cloudy and plants transpire mildly, but in Austin, rains are separated by day after day of intense sunlight with low humidity; thus, plants transpire rapidly. Soils in the Pacific Northwest are thick and organic and hold rainwater in place for days, whereas Austin’s soil is often less than 0.5 inch thick and it lies over porous limestone: Rainwater drains away immediately.
Water is water, but there are many ways in which it is available to plants, animals, and other organisms. Likewise, there are even more ways in which organisms have become adapted such that they can use this essential resource.

FIGURE B12-1C Cacti are not the only plants that survive drought by storing water in succulent bodies. The deserts of Africa and the Middle East have many succulent plants as well; this is Huernia recondita, in the milkweed family, and there are also succulents related to geraniums and to dandelions among others.
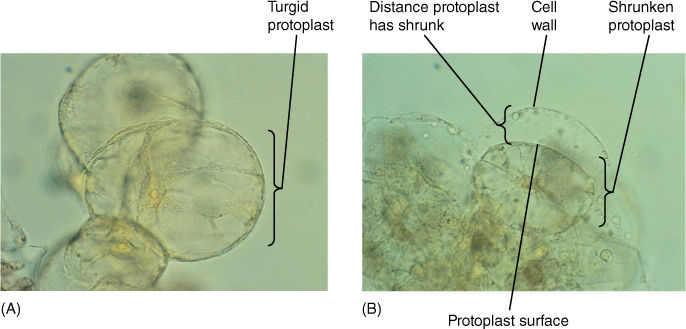
FIGURE 12-11 Some aspects of biotechnology processes require that the cell wall be digested away with cellulase enzymes, leaving behind a naked protoplast. The digestion mixture must contain just enough solute—usually sucrose or the sugar-alcohol mannitol—that the cells lose water and shrink away from the wall. If not, the protoplasts burst when the wall is removed. The solute concentration must be adjusted carefully; if too strong, too much water is pulled out of the cells and they die. (A) Cells in suspension culture before treatment. (B) Cells in 6% mannitol with the protoplasts just pulling back from the wall (both ×500).
Although plant cells cannot absorb so much water that they burst, water loss can be a serious problem. Imagine that our demonstration cell, now in pure water and with a water potential of 0.0 MPa, is placed in a strong sugar solution with a water potential of —2.0 MPa. Water moves out of the cell, osmotic potential becomes slightly more negative, and the pressure potential drops rapidly. In such a strong solution, long before the cell reaches equilibrium it loses so much water that the protoplast shrinks in volume and no longer presses against the wall. Plants never absorb so much water that their cells burst, but they frequently lose enough water to wilt because their protoplasts do not press firmly against the cell walls. The wall is not stretched and does not exert any pressure back; therefore, the pressure potential drops to 0.0 MPa. The point at which the protoplast has lost just enough water to pull slightly away from the wall is called incipient plasmolysis and is quite important (FIGURES 12-12 and 12-13). Up to that point, the cell has lost very little water, so its volume change and osmotic potential change have not been great, but because the pressure potential is now zero, the water potential equation is
ψ = ψπ + 0
If the cell has not reached equilibrium at the point of incipient plasmolysis, it continues to lose water, and the protoplast pulls completely away from the wall and shrinks. The cell has become plasmolyzed. Water potential continues to become more negative entirely because of the osmotic potential as solutes become more concentrated. Most plants at the equilibrium point of —2.0 MPa would die of severe water loss.
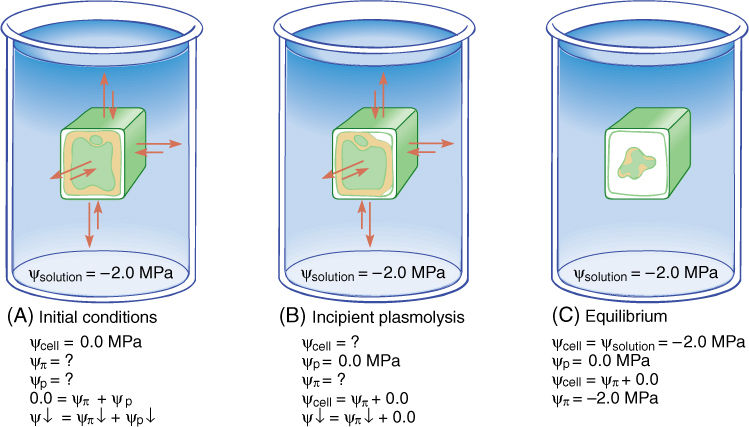
FIGURE 12-12 (A) When placed in a solution with a strongly negative water potential, the cell loses water rapidly and cell volume drops. ψp becomes less positive and ψcell becomes more negative; ψp changes only a small amount. (B) Incipient plasmolysis is the point at which the protoplast has shrunk just enough to pull away from the wall, and thus, ψp is zero and ψcell equals ψp. (C) If the cell does not reach equilibrium at incipient plasmolysis, it continues to lose water, and ψcell continues to become more negative until it reaches -2.0 MPa. The pressure potential here cannot become a negative number; therefore, the changing water potential is due to a changing osmotic potential. During plasmolysis, the cell loses enough water to change the concentration of solutes significantly.
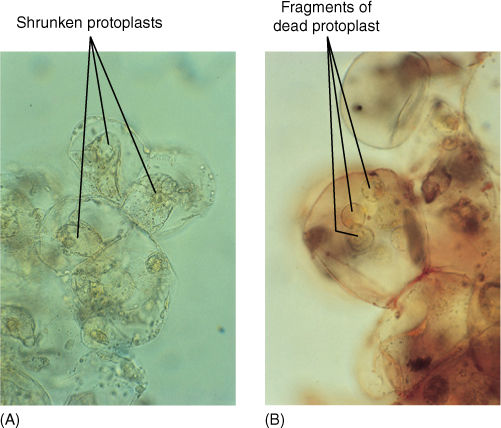
FIGURE 12-13 (A) These cultured cells have been placed in 12% mannitol for several hours and are severely plasmolyzed (compare with Figure 12-11). (B) After a few days of severe plasmolysis, the cells have died (both ×500).
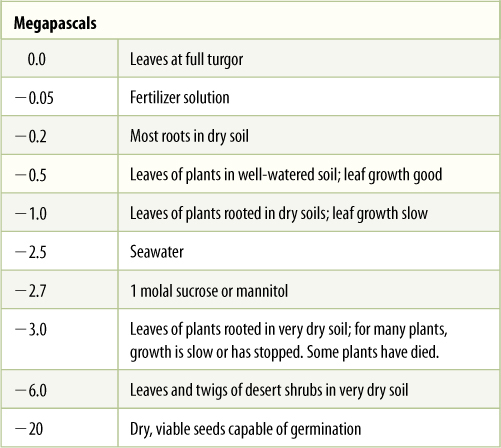
Although such severe desiccation kills most cells, some can survive it easily. The embryos in most seeds are much drier, having water potentials as low as —20 MPa. Less dramatically, the leaves of desert shrubs in dry soil have water potentials as low as —2.0 to —6.0 MPa (TABLE 12-2). For most plants of temperate climates, a leaf water potential below —1.0 MPa stops leaf growth, although leaves can survive such desiccation for many days or weeks.
TABLE 12-2 Water Potentials of Various Tissues Under Certain Conditions
![]() Short-Distance Intercellular Transport
Short-Distance Intercellular Transport
Most plant cells communicate with their neighboring cells, transferring water, sugars, minerals, and hormones at least. This movement occurs by a variety of mechanisms. First, all living cells are interconnected by plasmodesmata, the fine cytoplasmic channels that pass through primary cell walls. All of the protoplasm of one plant can be considered one continuous mass, referred to as the symplast.
Material also is transferred from one cell to another by transport across the plasma membrane. The methods involve osmosis, molecular pumps in the plasma membrane itself, or fusion between transport vesicles and the plasma membrane. Once across a plasma membrane, a molecule initially resides in the cell wall. The wall is probably thin and permeable, and the molecule can penetrate it easily, diffusing across it to an intercellular space or laterally through it, spreading along the cell surface (FIGURE 12-14). Most small molecules can move easily through both the wall and the intercellular spaces; the two together are called the apoplast of the plant.
In glands, the apoplast is mostly intercellular space through which molecules move easily, usually toward the surface of the gland. In nonglandular regions, the apoplast is mostly cell wall. The secreted molecule is probably absorbed by a cell neighboring the one that secreted it. In most parenchymatous tissues, primary walls are thin (less than 1 mm thick), and the contact faces between two cells are so extensive (10 to 20 mm2) that the probability is much greater that a molecule will either diffuse more or less directly into the next cell or re-enter the cell from which it came. If the molecule was originally secreted by active transport, the original cell membrane is probably impermeable to it, at least in that area, so return to the original cell is usually not possible. This is probably the most common mechanism for movement of water, sugar, and other nutrients between parenchyma cells within cortex, pith, or leaf mesophyll.
Guard Cells
The opening and closing of stomatal pores are based on short-distance intercellular transport. At night, when stomata are closed (except for Crassulacean acid metabolism [CAM] plants), guard cells are somewhat shrunken and have little internal pressure. They are in hydraulic equilibrium with surrounding cells: Water enters and leaves guard cells at approximately the same rate; no net change occurs in the amount of water. When guard cells must open, such as just after sunrise, potassium ions (K+) are actively transported from surrounding cells into guard cells (FIGURE 12-15). Once inside the guard cells, the potassium cannot leave because the plasma membrane is impermeable to it: Potassium pumping is possible but diffusion is not. The loss of potassium causes the water potential in adjacent cells to become less negative, whereas absorption of potassium causes water potential in guard cells to become more negative. Adjacent cells and guard cells are thrown out of hydraulic equilibrium by potassium pumping, and water diffuses out of surrounding cells across their plasma membrane, across the two primary walls and middle lamella, and into guard cells across their plasma membrane. The extra water and potassium cause guard cells to swell, bend, and push apart, opening the stomatal pore. After they are open, potassium pumping stops. Water movement brings guard cells and adjacent cells into water potential equilibrium again, and net water movement stops. When guard cells must close, the process is reversed: Potassium is pumped from guard cells into surrounding cells and water follows. Guard cells and adjacent cells are in equilibrium when stomata are fully open and fully closed.
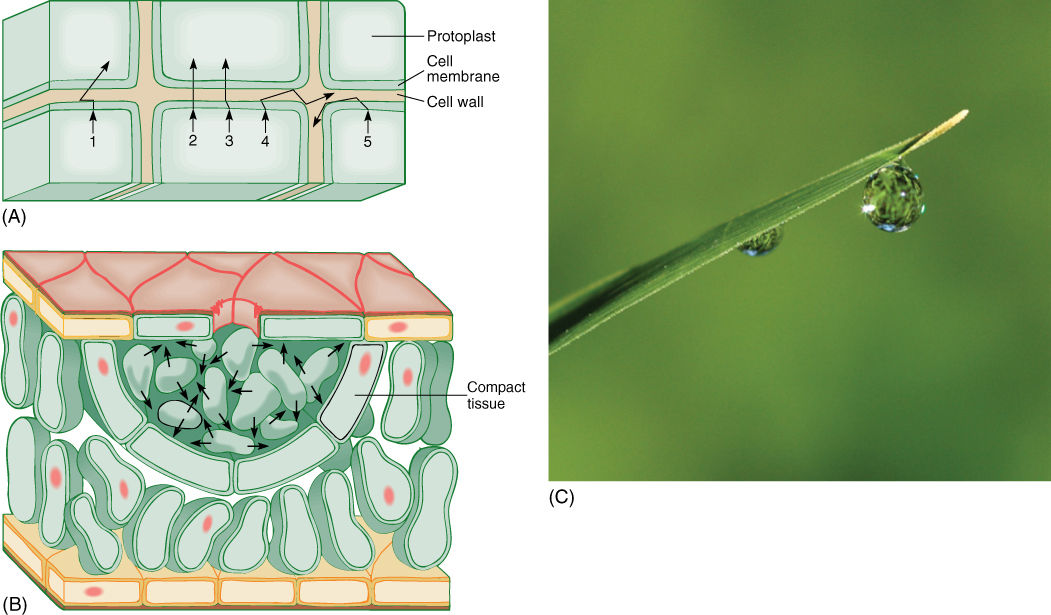
FIGURE 12-14 (A) After being released from a cell, a molecule diffuses in a series of random short paths as it collides with and bounces off other molecules. Probability favors its entering a neighboring cell (molecules 1, 2, and 3), but it can diffuse laterally along the wall as well (molecules 4 and 5). (B) In many glands, the apoplast is large, so movement between cells may be faster and easier than movement within cells. Such glands often have a lining of compact tissue that isolates the gland, preventing the secreted material from permeating the entire region. (C) This leaf tip of coastal sea oats (Uniola paniculata) is secreting water from a gland similar to that in (B).
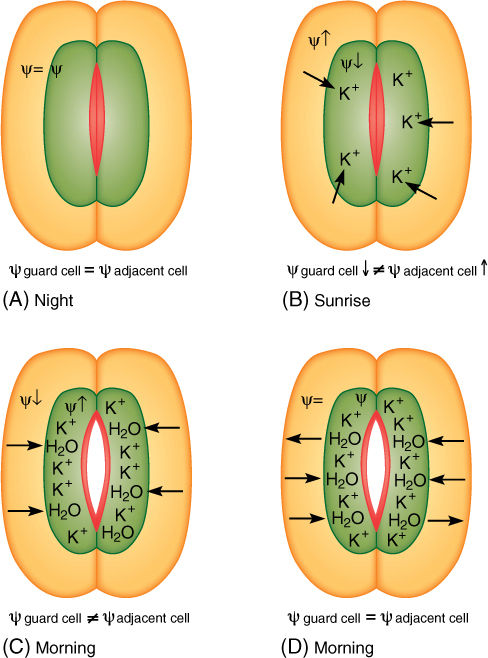
FIGURE 12-15 At night, guard cells and adjacent cells are in hydraulic equilibrium (A), but at sunrise, potassium is pumped into guard cells, increasing solute concentration (B). Osmotic potential and water potential become more negative, and water flows in (C), causing guard cells to swell and open the pore. As pressure builds, pressure potential rises, counteracting the falling osmotic potential; therefore, the guard cell’s water potential rises and moves back into equilibrium with the adjacent cell, but by the time that happens, the stomatal pore is open (D). At night, when the stomata must close, all steps are reversed.
Notice also that guard cells of fully opened and fully closed stomata are both in equilibrium with surrounding cells (and thus also with each other), even though they all have different internal conditions: When open, the guard cells’ abundant potassium gives them a very negative osmotic potential, which is countered by turgor pressure, giving a large pressure potential. This results in only a small negative water potential. When closed, the guard cells have less potassium; therefore, only a small negative osmotic potential. This is countered by less turgor and therefore a less positive pressure potential. These cells too have only a small negative water potential. Even though two cells might have very different internal conditions, they can have the same water potential and be in hydraulic equilibrium.
Plants and People
BOX 12-2 Farming “Wastelands”
It has been proposed that we develop plants that tolerate high levels of salt and use them as crop plants in arid, marginal “wastelands” where fresh water is scarce. This might be possible in two ways: We could examine desert-adapted plants to see whether any have useful properties such as nutritious seeds, medicinal drugs, or useful fibers. Alternatively, desert-adapted plants could be studied to identify which of their features make them drought resistant; then the corresponding genes could be identified, cloned, and transferred to some of our crops that are not now drought adapted. Once either of these types of plants is available, they could be grown in semidesert regions or perhaps even in deserts using a little irrigation with seawater or brackish water.
This concept has many problems. The first is that it is one thing to find plants with enough drought and salt tolerance to grow in seawater during a 1-year-long experiment, but it is another thing to irrigate an area with seawater year after year. Because they have little rain, most arid regions have only small, temporary rivers that have not been able to carve effective drainage channels: They often end in dry lakes with no outlet. After a rain, water is present for a few weeks, but it evaporates, leaving its minerals behind. The rivers do not carry minerals out to sea continuously as do rivers in the eastern United States and other moist areas. If seawater is used for irrigation in an arid area, tremendous amounts of salt would be deposited as the water evaporates. This would continue year after year until the salt concentration becomes so high that a salt desert is created, such as occurs in the Great Salt Lake in Utah, the Dead Sea in the Middle East, or the Devil’s Golf Course in Death Valley, where accumulated salt is so abundant it forms crystals 2 or 3 feet tall. Nothing could grow—not the new crop plants, not the genetically engineered plant, and certainly not the original plants, which would all be destroyed when the region was plowed to start the project. Even using fresh water rather than seawater causes a gradual accumulation of salt, and California’s Central Valley is already facing a serious problem of salt accumulation in the soil.
Another problem with this type of project is the concept that deserts and semideserts are wasteland, that they must be “developed” and “improved.” Although this type of thinking may have been popular at one time, many people now disagree with it. These areas, in their natural state, have an intrinsic worth. They are home to a great diversity of plants, animals, fungi, and other species. Even if none of these “wasteland” species is ever discovered to contain a medicinal drug or other useful feature, does that give us the right to exterminate them? Is it really necessary to bring more land into cultivation when so many Americans are overweight or eat foods that have been processed specifically to remove calories from them?

FIGURE B12-2 (A) This desert would be considered “wasteland” by some people, but it is where many rare and unusual plants live. This area has not only high biodiversity (there are many different species of plants, animals, fungi, and other organisms) but also peace, quiet, beauty, and a chance for people to reconnect with nature. The plants that look like upside-down green octopuses are ocotillos (pronounced oh ko TEA yoz; Fouquieria splendens). Fortunately, this “wasteland” is protected from “developers”: it is part of Big Bend National Park (B).

FIGURE 12-16 (A) Each leaflet of the compound leaf of Oxalis is joined to the petiole by motor cells; here they were photographed in the morning when it was cool, and the sunlight was not intense. Motor cells are turgid and leaflets are held into the sunlight. (B) Oxalis plants in full, intense sunlight. The motor cells have lost potassium and water; thus, they are not turgid. Leaflets hang down, minimizing their exposure to light. Later in the afternoon, when sunlight is not so intense, or if the shadow of a tree moves across the plant, the motor cells will absorb potassium, then water, and raise the leaflets again.
Motor Cells
The leaves of sensitive plant (Mimosa pudica), prayer plant (Oxalis), and many other species move slowly and reorient themselves by flexing and folding in response to a variety of stimuli (FIGURE 12-16). The location of flexure is either the entire midrib or the point at which the petiole attaches to the lamina or stem. The cells at these “joints,” called motor cells, are similar to guard cells: They can either accumulate or expel potassium and thus adjust their water potential and turgidity.
In Venus’ flytrap, the leaf can close rapidly, in less than a second, but it requires several hours to reopen. Motor cells are located along the midrib, and when they are shrunken, pressure in other midrib cells causes the two halves of the blade to be appressed, and the trap is closed. Trap opening occurs as potassium is slowly accumulated by motor cells, water diffuses in, and the motor cells become turgid. Closure is not caused by pumping potassium out of motor cells; that would be too slow. Instead, the membrane suddenly becomes freely permeable to potassium, and it rushes out instantly. The water balance is rapidly changed, and water too floods out, allowing the motor cells to virtually collapse; the trap then shuts quickly enough to catch insects.
Transfer Cells
The rate at which material can be actively transported depends on the number of molecular pumps present, which in turn depends on the surface area of the plasma membrane: The larger the membrane, the more molecular pumps it can hold. In certain specialized transfer cells, the walls are smooth on the outer surface but have numerous finger-like and ridge-like outgrowths on the inner surface. The plasma membrane is pressed firmly against all of the convolutions and thus has a much larger surface area than it would if the wall were flat. Consequently, room is available for many molecular pumps, and high-volume transport can occur across these transfer walls. Transfer cells are found in areas where rapid short-distance transport is expected to occur: in glands that secrete salt, in areas that pass nutrients to embryos, and in regions where sugar is loaded into or out of phloem.
![]() Long-Distance Transport: Phloem
Long-Distance Transport: Phloem
Although the exact mechanism by which water and nutrients are moved through phloem is not known, most evidence supports the pressure flow hypothesis. Membrane-bound molecular pumps and active transport are postulated to be the important driving forces.
The sites from which water and nutrients are transported are sources. During spring and summer, leaves are dominant sources, as their photosynthetically produced sugars are exported to the rest of the plant. At other times, such as early spring, before new leaves have been produced by deciduous trees, the sources are storage sites such as tubers, corms, wood and bark parenchyma, and fleshy taproots. Cotyledons and endosperm are sources for embryos during germination. Within sources of many species, sugars are actively transported into sieve elements—sieve tube members in angiosperms, sieve cells in plants other than angiosperms. In other species, phloem is loaded by the polymer trap mechanism: Conducting-cell plasma membranes are permeable to monosaccharides and disaccharides but not to polysaccharides. Simple sugars merely diffuse into the conducting cells and then are polymerized into polysaccharides that cannot diffuse back out. In both loading mechanisms, cells surrounding sieve elements, both companion cells and other phloem parenchyma cells, are important in loading phloem; many of the cells are transfer cells. It is common now to think of the functional unit as consisting of both a conducting cell and one or several companion cells, and the term STM/CC complex is used.
As sugars accumulate in sieve elements, the sieve element protoplasm becomes more concentrated (FIGURE 12-17A). Consequently, both its osmotic potential and its water potential become more negative. This causes hydraulic disequilibrium between sieve elements and surrounding cells, and water diffuses into the sieve elements. In any other cell, the increased volume of sugars and water would cause the protoplast to expand and press against the cell wall, but sieve elements are unique, being living cells with relatively large holes in their walls, up to 14 mm wide in cucumbers and pumpkins. When pressure starts to build in these cells, “protoplasm” is squeezed through the sieve pores into the next cell. Sieve element protoplasm is not like that of most cells: The vacuolar membrane (tonoplast) disintegrates, allowing vacuolar water to mix with part of the cytoplasm, creating an extremely watery, nonviscous substance—phloem sap. The majority of the protoplasm is held firmly to the walls, probably by microtubules or microfilaments, and is not carried away with the watery central phloem sap.
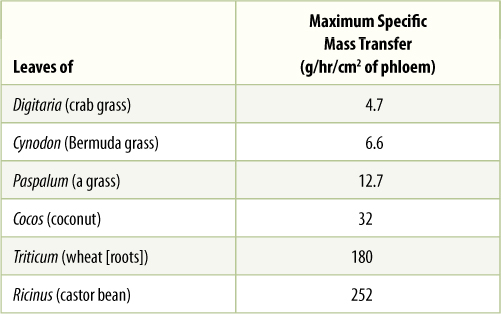
In sources, phloem loading occurs along numerous vascular bundles such as the fine veins in leaves, the network of bundles in tubers and corms, and the inner bark in storage roots and stems. With this massive loading, pressure builds quickly, and a large volume of material flows from the source. Pressures as high as 2.4 MPa have been measured in some sieve tubes (human blood pressure is about 0.016 MPa). The rate of transport can be high; up to 660 cm/hr has been measured in leaves of corn (TABLE 12-3). The actual amount of sugars and other nutrients (excluding water) transported by phloem per hour is called the mass transfer. Vascular bundles vary not only in the speed at which their phloem translocates but also in the amount of phloem present. The number of bundles leaving a source is also important. To make comparisons easier, mass transfer can be divided by the cross-sectional area of phloem to obtain the specific mass transfer (TABLE 12-4).
Sinks are sites that receive transported phloem sap, and they are extremely diverse. Storage organs are important in perennial plants during summer, but also important are meristems, root tips, leaf primordia, growing flowers, fruits, and seeds. On even a small tree there may be thousands of sinks, each receiving nutrients. Not all sinks are active simultaneously; most plants do not produce flowers and leaves at the same time, and fruits can develop only after flowers. Within sinks, sugars are actively unloaded from sieve elements into surrounding cells. The loss of sugar causes phloem sap to be more dilute, and its osmotic potential and water potential tend to become less negative; thus, water diffuses outward into the surrounding cells (FIGURE 12-17B). As a result, even though phloem sap flows rapidly into a sink, the end cells of the phloem do not swell. As quickly as nutrients are loaded at sources, they are unloaded at sinks.
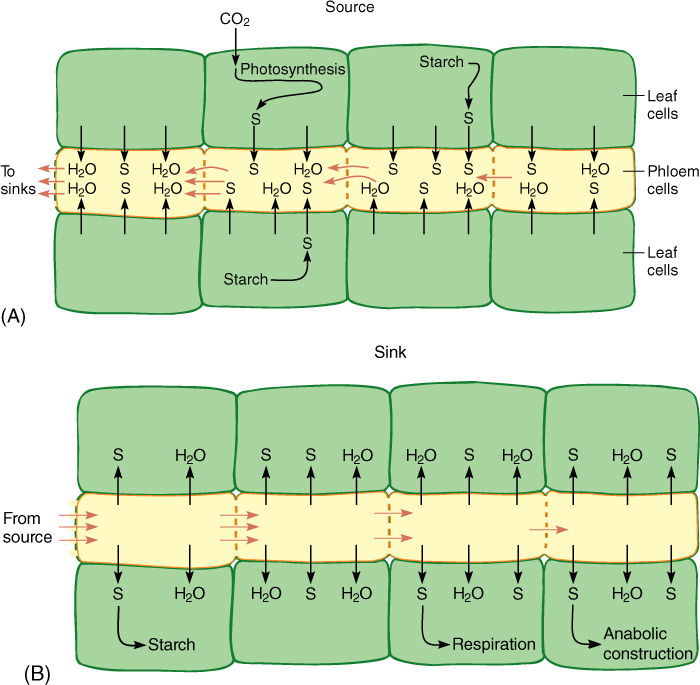
FIGURE 12-17 (A) As sucrose (S) is actively transported into sieve elements, ψp and ψcell become more negative, moving away from equilibrium with companion cells and other neighboring cells. Water moves into the sieve elements, squeezing phloem sap out through the sieve pores. Because the sugary water escapes through sieve pores, pressure does not build high enough to stop the influx of water. Water potentials of sieve elements and surrounding cells never reach equilibrium as long as sucrose is being pumped. (B) In sinks, sucrose is actively transported out of sieve elements, and all processes work in reverse compared with sources.
TABLE 12-3 Speed of Phloem Sap Translocation
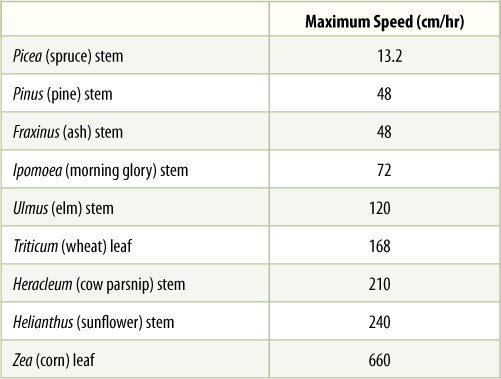
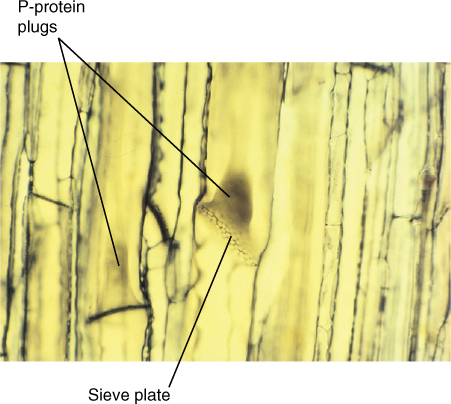
FIGURE 12-18 When this material of squash (Cucurbita) was being prepared for microscopy, it was cut open, causing the phloem sap to surge toward the cut, sweeping P-protein along and forming P-protein plugs, visible as dark brown masses. Sieve pores in the sieve plates are also visible (×500).
TABLE 12-4 Specific Mass Transfer
Because phloem sap is under pressure, the danger exists of uncontrolled “bleeding” if phloem is cut. Vascular bundles are broken open frequently, especially by chewing insects and larger animals. Two mechanisms seal broken sieve elements. The first is P-protein (P for phloem), found as a fine network adjacent to the plasma membrane inner surface of uninjured sieve elements. When phloem is ruptured, the phloem sap initially surges toward the break; this rapid movement sweeps P-protein into the cell center, where it becomes a tangled mass. When it is carried to a sieve area or sieve plate, the P-protein mass is too large to pass through and forms a P-protein plug (FIGURE 12-18). P-protein is present in all eudicots and many but not all monocots; conifers do not produce P-protein.
Within uninjured phloem there is another polymer as well, callose. Apparently, it stays in solution only if it is under pressure; when injury causes a pressure drop, callose precipitates into a flocculent mass and is carried along with the P-protein to the nearest sieve areas. There the callose contributes to the plug, and leaking is prevented.
In monocots with long-lived stems, such as palms and Joshua trees, sieve tube members live and function for many years, even hundreds of years, but in all other plants, individual sieve elements have a lifetime of only months or even weeks. They stop transporting and are replaced by new phloem cells from the provascular tissues or vascular cambium. After they cease to function, callose deposits seal them permanently.
A further aspect of phloem transport is important to consider. As sugar is actively transported into phloem in sources, what happens to the water potential of the cells losing the sugar? Shouldn’t it become less negative? No, it remains unchanged, because the sugars are being exported at the same rate they are being synthesized in leaves. Chlorenchyma cells absorb carbon dioxide molecules, but this does not cause the water potential to become more negative because the carbon dioxide is synthesized into sugar and exported. Millions of molecules of carbon dioxide may pass through a chlorenchyma cell without any long-term impact on its osmotic or water potentials. In sources such as tubers, sugar export has no impact on the storage cell water potential because as rapidly as it is exported, new sugar appears by depolymerization of starch. The same is true at sinks: Storage cells do not accumulate sugar as sucrose but instead polymerize it into starch. Thousands of molecules may be absorbed, but because they are polymerized into one molecule, no change in water potential occurs. In growing cells of sinks such as meristems and buds, the imported sugar is polymerized to cellulose, hemicellulose, and other carbohydrates. It can also be metabolized into amino acids, fatty acids, and nucleotides; these are then polymerized into proteins, fats, and nucleic acids, and thus, again, little or no change in osmotic potential and water potential occurs. In all cells, part of the imported sugar is respired, but this converts it to carbon dioxide that is expelled; so again there is no osmotic effect.
Plants control the direction and rate of flow of phloem sap. While dormant in late winter and early spring, buds receive very little phloem sap, but after they become active, phloem transport increases greatly. Not all buds are equally affected; some grow rapidly whereas others, even though located quite close by, receive virtually nothing. While flowers are open, phloem transport is low, but after fertilization, when the ovary begins to develop into a fruit, transport increases.
The direction of transport can also change. Leaf primordia and young expanding leaves are sinks; imported sugar allows them to develop much more rapidly than they would if they had to be completely autotrophic (see Figure 12-2). In addition, they also need large amounts of nitrogen, sulfur, potassium, and other minerals. After leaves reach a critical size, they become self-supporting, able to photosynthesize rapidly enough to meet all their own needs (FIGURE 12-19). Shortly afterward they become sources, exporting material. Phloem transport is reversed in the leaf and petiole; molecular pumps must now load the phloem, not unload it. Plasma membranes may be altered, or one set of sieve elements may cease to function and may be replaced by an entire new set of cells. The early primary phloem often lives for less than a few weeks.

FIGURE 12-19 This bean contains two prominent sources, the cotyledons, which are supplying sugars, amino acids, and minerals to the rest of the seedling. The shoot tip with its meristem and leaf primordia are sinks, as are the roots. The first two leaves are expanded and are probably sources now, but they were sinks while they were developing.
Leaves become self-sufficient and stop importing sugars when they have grown to about one-quarter of their mature size. At this point, the minor veins have not yet formed, so phloem unloading must occur by higher order veins, not by the minor veins. As leaves continue to mature, minor veins are finally established, and the phloem in them is then used for export of material out of the leaf.
Leaves near a stem tip export upward to the shoot apical meristem, while leaves farther back export toward the trunk and roots. As the shoot apex grows, leaves that had been near the apex are left behind and their transport shifts from upward to downward. If the apex produces flowers and then fruit, the direction of transport may shift again so that all leaves send sugars upward.
![]() Long-Distance Transport: Xylem
Long-Distance Transport: Xylem
Properties of Water
The movement of water through xylem is based on a few simple properties of water and solutions. One property is that water molecules interact strongly with other water molecules, behaving as if weakly bound together; when frozen, the molecules become strongly bound to each other. Because of this, liquid water is said to be cohesive, and any force acting on one molecule acts on all neighboring ones as well.
Another property of water is that its molecules interact with many other substances—it is adhesive. Almost all substances in plants, except lipids, interact with water: Cellulose, enzymes, DNA, sugars, and so forth have a shell of water molecules rather firmly attached to them. Occasionally, a water molecule vibrates out of one of these shells and is replaced by another water molecule, but in general, adhesion makes these water molecules less free to move around than other water molecules.
Water also adheres firmly to soil particles. When soil is quite moist, roots can absorb the free liquid water between soil particles, but as the soil dries, the remaining water adheres firmly to the soil and cannot be absorbed easily, if at all (FIGURE 12-20). Even though the soil may contain considerable water, it is unavailable to the plant. The same is true of seawater; water molecules interact so strongly with the salt molecules that land plants cannot pull the water away.
Another property of water is that it is heavy, and lifting it to the top of a tree requires a great deal of energy. If water were lighter, less energy would be involved.
Water Transport Through Xylem
Water movement through xylem and plants as a whole is governed by the principles of water relations just described. The cohesion-tension hypothesis is the most widely accepted model of the process. When stomatal pores are open, they unavoidably allow water loss. The apoplastic space of spongy mesophyll and palisade parenchyma is filled with moisture-saturated air, so water molecules have a strong tendency to diffuse from intercellular spaces to the atmosphere. Even relatively humid air has a tremendous capacity to absorb water: At 50% relative humidity, warm air can have a water potential as negative as -50.0 MPa. This water loss is called transstomatal transpiration. The cuticle and waxes on the epidermal surfaces are fairly efficient isolation mechanisms, being so hydrophobic that very little water passes through them; however, some water is lost directly through the cuticle by transcuticular transpiration (TABLE 12-5).
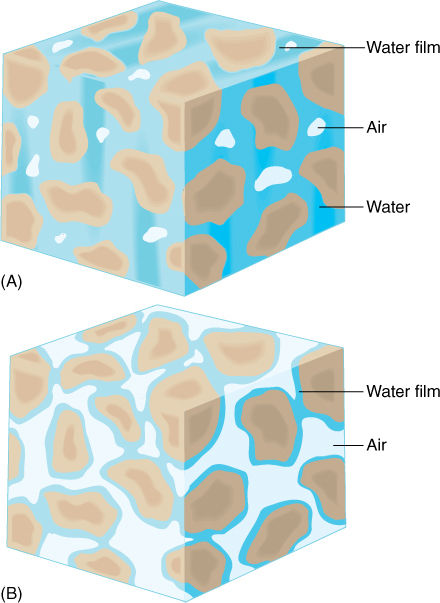
FIGURE 12-20 Wet soil contains water both as a film covering all surfaces of soil particles and as small masses held in capillary spaces formed where soil particles touch (A). The latter is held weakly and can be easily absorbed by roots. (B) Dry soil contains only tightly bound films of water. This adhesion is measured by ψm, which can be so negative that the soil’s water potential is also very negative. This water cannot be absorbed by the roots of most plants.
Consider a leaf in early morning: Stomata are closed, air is cool, and relative humidity is high. The air may have cooled enough during the night to allow dew to form. Cells within the leaf are turgid and probably in equilibrium with each other, all having a water potential between 0.0 and —1.0 MPa (see Figure 12-7). As the sun rises, stomata open and begin losing water; the air warms, and its relative humidity decreases. As transpiration causes epidermal cells and mesophyll cells near stomata to lose water, their water potentials become more negative, going out of equilibrium with surrounding cells. The disequilibrium does not become major because water diffuses into these cells from other cells and apoplastic spaces deeper within the leaf. But this water movement out of the deeper mesophyll cells causes their water potentials to become more negative, away from equilibrium with even deeper cells (FIGURE 12-21).
TABLE 12-5 Transstomatal and Transcuticular Transpiration*
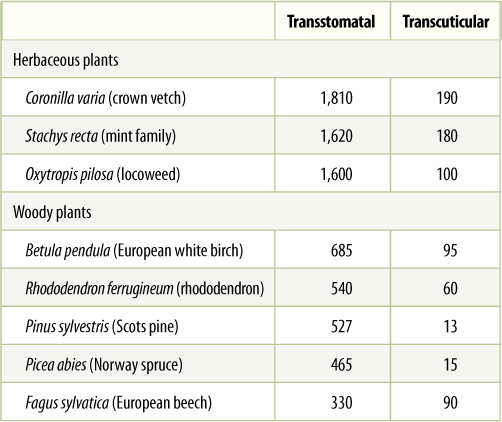
* Rates are mg H2O/dm2/hour (dm is decimeters; 1 dm = 10 cm). Surface area includes both sides of the leaf.
Finally, this gradient of water potentials reaches a tracheid or vessel member. As water molecules move out of tracheary elements into mesophyll parenchyma cells, the water potential within the xylem water column becomes more negative. The loss of water from tracheary elements does not really affect the xylem osmotic potential because solutes are very dilute to begin with. Here water’s cohesive properties are more important: As a water molecule leaves the xylem, it does not leave a hole behind but instead drags other water molecules along with it. All water molecules of the plant are hydrogen bonded together, but the water molecules in the xylem can move upward most easily. That water is purest, is not bound to proteins and cellulose, is not locked into hydration shells around solutes, and so on. As water molecules diffuse out of xylem in the leaves, cohesive forces pull water upward through the xylem, all the way from the roots (Table 12-5 and TABLE 12-6). Think of an icicle: If the top molecules are pulled upward, the entire mass of icicle is lifted.
Water is heavy, and water molecules in the uppermost tracheary elements must lift the weight of the entire water column. There is tension (pull) on these molecules, and consequently, the pressure potential is a negative number; as water moves into the leaf mesophyll, the xylem water potential becomes more negative because of an increasingly negative pressure potential. In vertical stems, water must move directly upward in the xylem, and the water’s weight is a significant consideration. In the examples discussed earlier, water could move laterally between a cell and a beaker; therefore, no lifting was involved. In vertical xylem, the weight of water counteracts its tendency to rise into areas of more negative water potential. Consequently, if leaf xylem water potential is only slightly more negative than root xylem water potential, the water does not move. For every 10 meters of height, leaf water potential must be at least 0.1 MPa more negative than root water potential (FIGURE 12-22). In trees such as elms and sycamores that are typically more than 30 meters tall, leaf water potential must be at least 0.3 MPa more negative than root water potential simply to overcome the weight of water. This is accomplished automatically: When stomata open in the morning, leaf cells lose water, and their water potentials become more negative; however, water does not begin moving upward in the xylem until drying causes the water potential of leaf cells to become sufficiently more negative. Stolons, rhizomes, and horizontal vines have no such problem; long-distance transport is horizontal and no lifting is involved, so gravity is not a factor. A few plants grow as pendant epiphytes, their stems dangling down from the branches of the host plant. Their stems and leaves are lower than their roots, and gravity assists water movement (FIGURE 12-23).
Water is extremely adhesive, and its molecules interact strongly with the polymers of the cell walls of tracheids and vessel elements. Water molecules adjacent to the walls tend to remain fixed to the walls and also tend to prevent neighboring water molecules from being drawn upward by transpiration/cohesion. This results in a layer of relatively immobile water that does not move easily. In narrow tracheids and vessel elements, this immobile water is a significant fraction of the water column. The resulting friction hinders water’s movement and contributes to the tendency of water to remain stationary even when leaves have a more negative water potential than roots. Imagine lifting an icicle: You must pull against its weight and the friction of the icicle in a tube (the cell walls), but lifting the top of the icicle raises the entire water column unless it breaks. As a rough approximation, to overcome friction, leaf water potential must be at least 0.1 MPa more negative than root water potential for every 10 meters of height; therefore, considering both friction and gravity, a difference of 0.2 MPa is needed for every 10 meters of height. In plants with numerous wide vessels, friction is less, and less than 0.2 MPa is needed; however, in plants with narrow tracheary elements, even more than 0.2 MPa is necessary. Also, in plants that have only tracheids, water molecules must be pulled through pit membranes when entering and leaving each tracheid, further contributing to friction.
Alternatives
BOX 12-3 Desert Plant Biology
Plants in many habitats have more than enough water; their leaves do not wilt, and they do not abort flowers or fruits because of insufficient water. Plants adapted to deserts, however, must survive periods when moisture is so scarce that more water is lost from the plant than is taken in. What alternatives make life possible in dry regions, and what are the consequences of each alternative?
What do we mean by “desert” or “dry environment”? Dryness results from the balance between precipitation (rain, snow, dew, and so on), evaporation, and soil texture. Precipitation may fall evenly throughout the year or seasonally, often as winter snow or summer thundershowers. In some habitats, rain occurs as drizzle lasting for days or weeks and is accompanied by cloudy, cool weather; in others, it comes torrentially, with 2 to 8 inches falling in a single day, followed by bright sunny weather. Periods without precipitation might last just a few weeks, but it is not unusual for rainless periods to last months in the Chihuahua, Sonora, and Mohave Deserts of the southwestern United States.
Evaporation is related to the relative humidity of air. Hot air can hold enormous amounts of water vapor; cool air can hold less. Precipitation occurs when air cools so much that its holding capacity drops below its actual moisture content: Excess water vapor condenses as fog, dew, rain, or snow; however, even cold air can be dry (think of chapped lips and dry skin in winter), and hot air can be humid (think of muggy summer days). Plants face water problems when periods of low precipitation coincide with periods of dry air.
Texture affects a soil’s capacity to store precipitation. Rocky, sandy soils, especially those on slopes, hold almost no water and become dry just days after a heavy rain. Fresh lava flows in Hawaii are deserts despite receiving rain almost every day. Alternatively, fine soils with some clay and abundant humus hold large amounts of water for months. Roots have a steady supply to draw from, but even this is a subtle complexity for plants: Sandy desert soils are actually beneficial because they allow even a light rainfall to penetrate down to the root zone. If the desert had rich soil with clay and humus, light rains would be held in the uppermost layers, leaving roots dry.
Averages are not especially helpful in understanding deserts and desert-adapted plants: Variations from year to year are important. The vegetation of any desert is the result of plants surviving not merely the droughts of average years but also rare protracted droughts that last several years. For example, cacti dominate the deserts of Mexico and the American southwest because they survive exceptionally long droughts that kill off any plant that can only survive average droughts. If every year were an “average year,” these plants would become so abundant that they would overgrow the cacti, shading them and ultimately killing them.
Two alternatives by which plants adapt to dry habitats are drought avoidance and drought tolerance. Most deserts have either a brief period when they are moist or they have small areas where water collects. Many drought-avoiding plants are known as desert ephemerals (ephemeral means short lived). They are small plants that complete their life cycle in just a month or two: Seeds germinate, seedlings grow, the plant flowers, produces new fruits and seeds in just a few weeks, while the soil is moist after a rain or a snow melt. By the time drought arrives, the plants have died, but their seeds are ready for the next moist season. These plants avoid dry conditions. Two consequences are that these plants (1) can live in deserts and (2) can never become large or perennial.
Drought-tolerating plants live through dry periods, losing water more rapidly than they gain it. Most accomplish this by being succulent: A high percentage of their body consists of water-storage cells. Stem succulents such as cacti, euphorbias, and stapeliads store water in pith and cortex. Leaf succulents such as agaves, yuccas, echevarias, and lithops have very thick leaves. Desert-adapted bulbs are leaf succulents in which the upper portion of each leaf extends above ground and is thin and photosynthetic, whereas the lower portion of each leaf is subterranean, thick, fleshy, and able to store water and nutrients through adverse periods. Desert-adapted bulbs survive for years in a dormant state, not producing any aerial leaves until moisture is sufficient. Root succulents are less common, but they include plants like yams (Dioscorea). Root succulents typically have nonsucculent shoots and leaves that die back during drought, being replaced in the next moist season by new shoots that sprout from the “root crown,” a bit of shoot located at the top of the root. Wood succulents produce wood with a high percentage of parenchyma cells or a type of fiber that stores water; examples are baobab trees (Adansonia grandidieri), boojum trees (Idria columnaris), and elephant trees (Bursera microphylla).
Each of these alternatives has particular consequences. Water stored in wood is near vessels and may be especially effective in preventing cavitations, but it must then be transported out to leaves and flowers as in nondesert plants. Succulent leaves store water near photosynthetic tissues, keeping them hydrated and photosynthesizing even during very dry periods. Stem succulents may have thin, flat ordinary foliage leaves that are ephemeral, abscising when dry seasons start, after which the stems must perform all photosynthesis. Other stem succulents, such as cacti, never have large foliage leaves and must rely solely on their stems for photosynthesis; such plants are always good at conserving water but can never take full advantage of occasional moist periods.
A completely different way of tolerating drought is the capacity to survive protoplasmic desiccation. Many desert-adapted mosses have no capacity to store water, and they are perennial, not ephemeral. As the habitat becomes drier, so does their protoplasm until they become dormant for months. The water potential of their cells becomes extremely negative, which would kill most plants, but these are merely inactive, not dead. On days with sufficient dew, fog, or light rain, the mosses rehydrate in just an hour or so and quickly resume ordinary metabolism, including photosynthesis, growth, and development. Their activity will last as long as the environment remains sufficiently moist, but this might be just a few hours. Having a body water content that changes with habitat moisture is called poikilohydry (cold-blooded animals are poikilothermic)—it also occurs in some liverworts, some species of Selaginella (“resurrection plants”), the tiny cactus Blossfeldia, and a few other vascular plants. Lichens are arguably the supreme examples of poikilohydry, being capable of drying to very low water content for months without dying, even if growing on rocks exposed to full summer sun.
TABLE 12-6 Speed of Xylem Sap Translocation
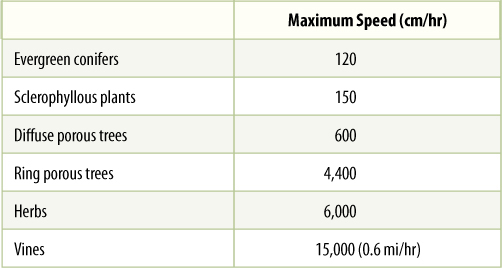
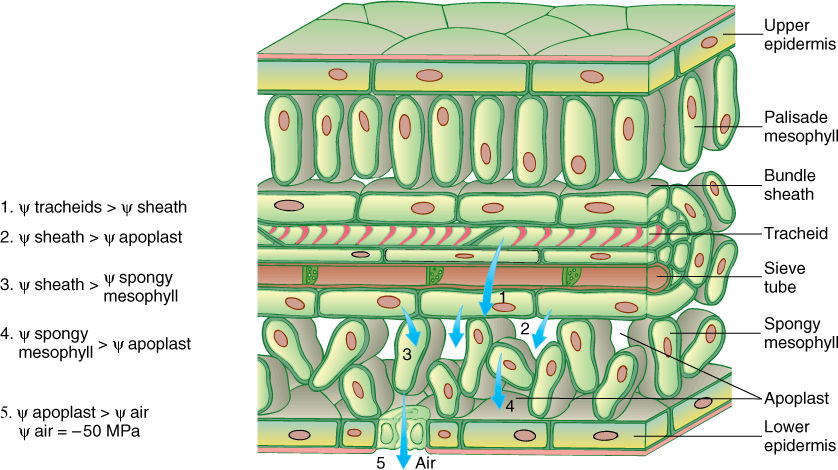
FIGURE 12-21 As water moves out of the leaf into the air, the tissues dry and a water potential gradient becomes established. Water flows from the xylem, where water potential is least negative, toward air, where water potential is most negative.
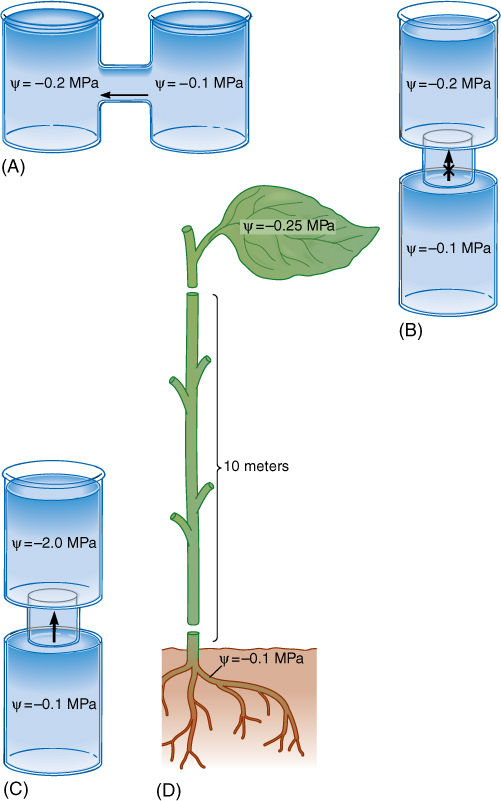
FIGURE 12-22 (A) If water can move laterally between two solutions, no lifting is involved. The slightest difference in water potential results in water movement. (B) If water must move upward against gravity, work must be done. A slight difference in water potential may not be enough to cause water movement; only a large difference will (C). (D) To overcome gravity and friction, the water potential of plant tissues receiving water must be at least 0.2 MPa more negative than that of roots for every 10 meters of height separating them. In the case illustrated here, with a difference of 0.15 MPa, water would not move up the stem. The water potential of the leaf would have to become 0.5 MPa more negative.

FIGURE 12-23 This epiphytic cactus (Rhipsalis) grows upside down. Its roots cling to the bark of a big branch of a rain forest tree, and its slender stems dangle straight down. Because its transpiration surfaces are lower than its roots, water flows downward from the roots, and gravity actually assists xylem conduction rather than hindering it, as in most plants.
Returning to the plant in our example, transpiration causes leaf cells to lose water, and their water potentials become more negative; water moves into them from tracheary elements, and tension pulls on water molecules in the xylem. When the water potential in the uppermost tracheary elements has become sufficiently more negative than that in the lower elements, friction and gravity are overcome and water moves upward. This causes the lowermost xylem cells to pull water inward from the root cortex, which in turn pulls water in from the root epidermis. Water potential of the root epidermis and root hairs becomes more negative than that of the soil, and water moves automatically into the root.
Long-distance water transport occurs in this manner as long as the soil is sufficiently moist. A sandy soil that has 30% moisture has a water potential of approximately —0.001 MPa, almost equal to that of pure water (TABLE 12-7). Water is held in soils by cohesion and adhesion as wedges and droplets between soil particles, and in sandy soils, root hairs can easily draw water from moist soils. Gravity and evaporation also pull water away from the droplets in sandy soils, so these soils dry quickly after a rain. As the soil dries, the most mobile molecules are removed, and those tightly bound to soil particles remain (Figure 12-20). In a dry soil, not only is less water present, but it is also relatively immobile. Clay soils are composed of thin flakes with a high surface-to-volume ratio. When wet, they hold a great deal of water, but it is firmly bound as a hydration layer. No root can pull water away from clay that is even slightly dry. Loam soils consist of sand, silt, and clay and have a diversity of pore sizes. During a rainfall, loam soils absorb large amounts of water and then hold it for weeks after the rain has stopped. The diversity of pore sizes allows root hairs to absorb water but prevents gravity from pulling the water so deep into the soil that roots cannot reach it.
TABLE 12-7 Water Potentials of Soils
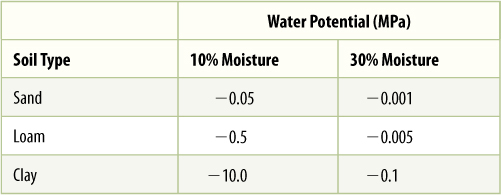
Many roots remain healthy with their water potentials as low as —2.0 MPa; they still absorb water from soils that are quite dry (FIGURES 12-24; see Table 12-2), but if the plant is 10 meters tall, the leaves would have to have a water potential at least slightly more negative than -2.2 MPa to overcome friction and gravity. This is also possible in some species, but typically, the leaves would either be dormant or preparing for abscission.
When both soil and air are dry, plants are greatly stressed. Even if stomata close, transpiration continues, at least through the cuticle. Leaf water potentials become more negative, but water cannot move upward easily because the soil is so dry. Tension on the water columns increases, and at some point, cohesion is overcome: Hydrogen bonding is broken over a large region, and the water column breaks. This breaking is called cavitation, and the water column acts just like a broken cable. Molecules above the cavitation point are drawn rapidly upward because they are now free of the weight of the water below them; those below the cavitation point rush downward because nothing supports their weight. Between the two portions is space called an embolism (often called an air bubble), which expands until its surface encounters a solid barrier such as a pit membrane. The water/embolism surface cannot pass through pit membranes, but it can pass through perforations because they are open holes (FIGURE 12-25). When an embolism forms in a tracheid, only that tracheid loses its water, but when an embolism forms in a vessel element, it may expand through perforation after perforation until the entire vessel has been emptied.

FIGURE 12-24 (A) Many desert shrubs withstand severe desiccation; even though their water potentials become extremely negative, the cells survive, although they may become inactive. This Nolina parryi is in full bloom despite its very dry habitat, Joshua Tree National Park. (B) Plants such as this floating aquatic stream plant (Ludwigia) do not tolerate water stress at all; if their water potential falls very far below —0.2 MPa, they die quickly. (C) During a drought lasting 8 months with no rain at all when this photograph was taken, the ground became so dry the bamboo died. However, the trees in the background were still healthy, and a few vining weeds climbing the dead bamboo were not merely surviving but actually growing.
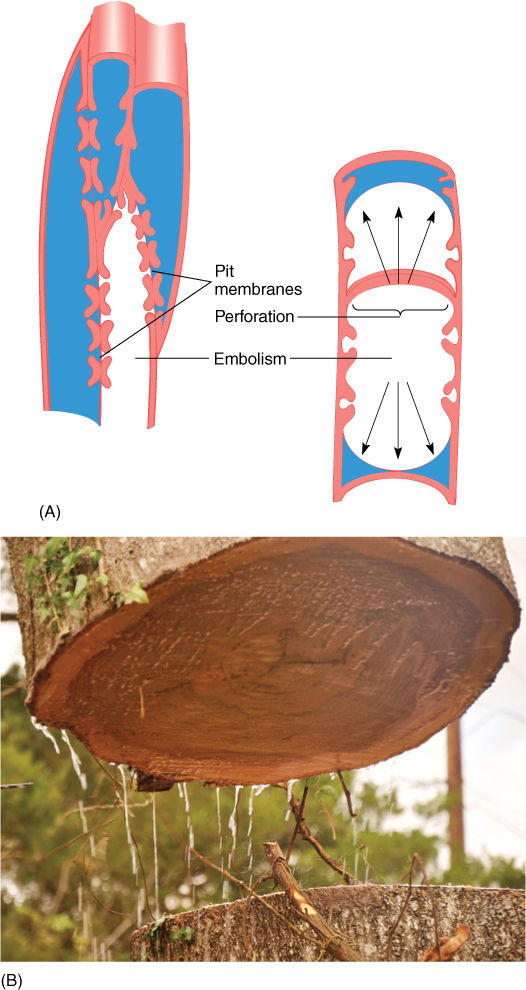
FIGURE 12-25 (A) Severe tension can overcome the cohesion of water molecules and cause an embolism to form and expand rapidly. The embolism can pass through holes such as perforations but is stopped by pit membranes. If an embolism occurs in a tracheid, it cannot spread beyond the tracheid, but if a vessel element cavitates, the embolism spreads throughout the entire vessel. (B) This is the trunk of a large water oak (Quercus nigra) that was cut down. As the chainsaw cut through the xylem vessels the water in them was cavitated, so now there is no hydrogen bonding to hold the water in the wood. The water here is pouring out of the wood, not just dripping, and the flow continued for almost a full minute. If this wood had been composed of tracheids, almost no water would have come out.
Cavitation often means that that tracheid or vessel can never conduct water again. The cohesive bonding that permits leaf transpiration to draw water upward has been disrupted. Under unusual conditions, embolisms are occasionally “healed.” If all of the surrounding cells are full of water and if the night is so cool and humid that transpiration stops, enough water may seep into the embolism to fill it and re-establish a continuous water column. More typically, any water that seeps in simply flows down the side of the tracheary element but is not able to fill it.
Because cavitation destroys the usefulness of an entire vessel or tracheid and because a plant invests considerable energy and reduced carbon in making tracheary elements, features that minimize cavitation are selectively advantageous. Adhesion between water and the cell wall is just such a feature, giving the water column extra strength so that it does not cavitate easily. This is most effective in narrow elements, where the reinforcement affects all water molecules, even those in the center. In wide elements, the central molecules are freely mobile and cavitate almost as easily as pure water. To clarify, cavitation breaks hydrogen bonds in water but does not damage cell walls at all (remember that tracheary elements are dead when conducting); all tracheary elements in heartwood are cavitated, but the wood is still strong.
This wall-induced reinforcement of water columns is believed to be the feature that allows plants to reach the heights they do. Redwood trees in northern California and southern Oregon are the tallest plants known; they grow to 100 meters in height (the record is 115 m—379 feet—for a tree named Hyperion), and water is pulled upward the entire distance through their tracheary elements. This cannot be duplicated with glass or metal capillary tubes; the water columns are too fragile to support their own weight without the reinforcing that cell walls provide. One hundred meters appears to be the limit for xylem; even with reinforcement, the cohesive forces at the top of the water column cannot support the weight of 100 meters of water hanging from them.
Some remarkably exquisite types of wood have evolved that are elegantly adapted for the various conditions. In the moist tropics, water is always abundant; thus, the soil is never dry, and water always moves easily. Reinforcement is not necessary, and the wood is full of wide vessels (FIGURE 12-26). In drier temperate areas, especially rocky slopes, water is frequently scarce, and water stress common. It is selectively advantageous for plants in such an environment to have narrow vessels or even wood with tracheids only, as conifers have. In temperate areas with good rainfall, plants usually have a moist spring and produce early wood (also called spring wood) with large vessels; the summer is drier, and they then produce late wood (summer wood) with narrower vessels or only tracheids. During the summer, the wide vessels of the early wood cavitate, and conduction occurs primarily or entirely in the late wood (FIGURE 12-27).
Eventually, all vessels and tracheids cavitate. Dry conditions in summer cause many cavitations, as do freezing in winter, vibration in wind, and damage by burrowing insects. After tracheary elements cavitate, surrounding parenchyma cells may block them off with tyloses or by secreting gums and resins. As more tracheary elements cavitate in a region of wood, adjacent parenchyma cells synthesize antimicrobial compounds then die, and the region becomes part of the heartwood.
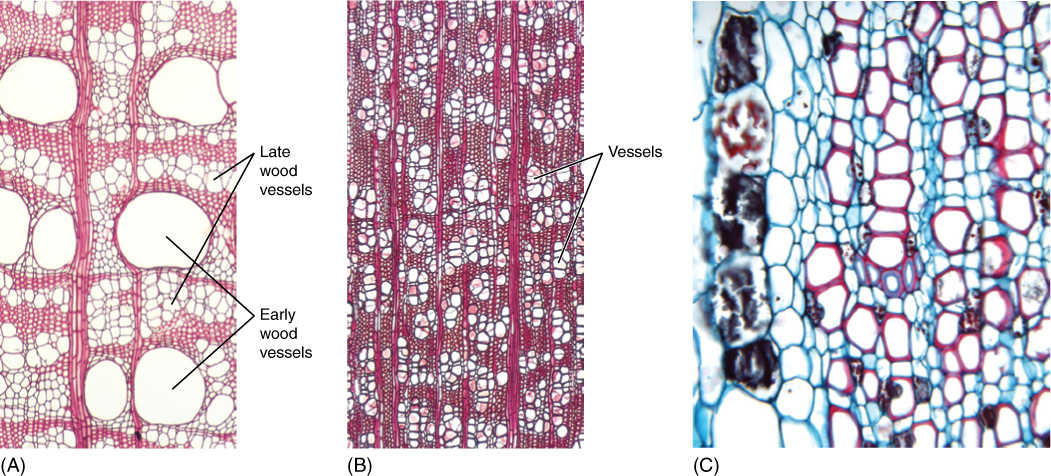
FIGURE 12-26 (A) Wood from a tropical tree. There are many broad vessels in the early wood, each of which can conduct water rapidly from the moist soil. Late wood has narrower vessels (×100). (B) This wood is from a tree of temperate climates; the vessels are narrow and abundant. No single vessel can conduct very much water, but if one cavitates, only a small fraction of the conducting capacity of the wood is lost (×100). (C) This is wood of a succulent relative of geranium, Pelargonium carnosum. Broad, open red cells are vessels. Narrow red cells are fibers (only about five are present), and all cells with thin blue primary walls are wood parenchyma cells. This plant stores water in its wood, minimizing chances of cavitation (× 200).
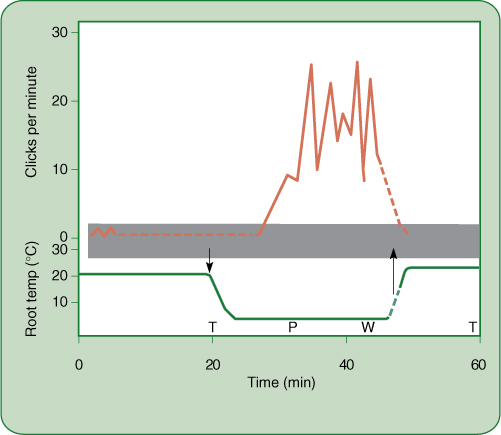
FIGURE 12-27 Cavitation of vessels and tracheids causes audible clicks that can be heard by sensitive microphones. This graph shows the induction of cavitation in castor bean xylem; plants were grown in water solutions that permitted water uptake to match transpiration loss—there was little tension on xylem water columns, and no cavitations occurred. Then the roots were cooled (arrow at left) to 5°C to inhibit root absorption of water, but shoots were kept warm to encourage transpiration. Water columns were stretched and immediately began breaking. After 20 minutes, embolisms were forming at almost 30 per minute, and the plants were wilting. At 45 minutes, the root solution was warmed to permit water absorption, and cavitation quickly stopped.
Control of Water Transport by Guard Cells
Bulk water movement through xylem is influenced and powered primarily by water loss to the atmosphere. Although water loss through the cuticle is important, transstomatal transpiration is more significant whenever stomatal pores are open. Open stomata represent a trade-off between carbon dioxide absorption and water loss. Whenever water supply in the soil is adequate, water loss is actually advantageous—water movement is the primary means of carrying minerals upward from roots to shoots, and the evaporative cooling that results from transpiration can prevent heat stress in leaves and young stems; however, if the soil is too dry to supply water, transpiration represents an immediate, potentially lethal threat due to desiccation. Numerous mechanisms have evolved that control stomatal opening and closing. Each mechanism is keyed to a particular environmental factor, and their interaction results in great sensitivity to potential stresses in the habitat.
If the leaf has an adequate moisture content, light and carbon dioxide are the normal controlling factors. For most healthy, turgid plants, light most often controls guard cell water relations. Blue light is the important, triggering wavelength, and the action spectrum of opening closely matches the absorption spectrum of a flavin or flavoprotein pigment. It is not yet known how absorption of light by the pigment leads to potassium pumping.
The presence of light also leads to photosynthetic fixing of carbon dioxide; the decrease in internal carbon dioxide concentration may also lead to stomatal opening. Artificial manipulation of the amount of carbon dioxide available can stimulate guard cells to open or close in light or dark. At night, with no photosynthesis, carbon dioxide levels are high and presumably contribute to stomatal closing.
All of these mechanisms in healthy plants are completely overridden by a much more powerful mechanism triggered by water stress. As leaves begin to dehydrate, they release the hormone abscisic acid. This hormone immediately causes guard cells to close the stomatal pore even in blue light and low concentrations of carbon dioxide, factors that would otherwise favor opening. Water stress-induced closure often occurs in the early afternoon on a warm, dry day if root uptake and xylem conduction cannot keep up with transpiration. Stomatal closure prevents carbon dioxide uptake and stops photosynthesis even though light is available.
In plants with CAM, stomata open at night and close in the morning. Temperature is particularly important for these plants; if night temperatures are too high, stomata may remain closed for days or weeks at a time. The low night temperatures typical of their desert habitats are essential for stomatal opening and carbon dioxide absorption. Under conditions of mild temperatures and abundant moisture in the tissues, such as after a spring rainfall, CAM plants convert to C3 metabolism, opening their stomata in the morning and picking up carbon dioxide with RuBP carboxylase directly. When the soil dries after several days, they revert to CAM and night opening of stomata.
 At the Next Level
At the Next Level
1. Wet plants produce dry seeds. There are many instances in which dry structures are produced by wet cells and tissues. Some processes are well understood, others are not. The polymerization of glucose into starch grains excludes water, producing dry starch in wet cells. But we do not understand larger processes very well. For example, after a flower has been open for a day or two, a plant blocks the flow of water to the petals and stamens even though they are healthy, and at the same time it maintains water flow to the carpels, ovules, and developing seeds. Later, seeds and some fruits become extremely dry even though the twig supporting them has plenty of water. The processes by which this is controlled are unknown; perhaps tyloses block xylem and tylosoids block phloem (tylosoids are similar to tyloses). Perhaps xylem and phloem parenchyma are involved.
2. Harvesting Agave sap for pulque. The alcoholic beverage pulque is produced by fermenting sap from several species of agaves. These are large desert monocots with giant, sharp leaves clustered around a short stem. To obtain the sap, harvesters wait until the plant is about ready to produce a giant inflorescence, then they cut the bud off and throw it away: The plant remains alive and produces abundant sap (called aguamiel), as much as 5 to 8 L per day. The sap oozes out for many days—in exceptional cases as long as a year—and each day the harvesters scoop up the sap and ferment it. This is an unusual example of a plant unable to control damage to its long-distance transport system. Perhaps the phloem does not produce enough P-protein and callose, perhaps the sap is xylem sap.
3. Refilling empty vessels after cavitation. After a vessel cavitates, it contains only air and is useless for conduction. Plants are highly redundant, having many vessels rather than just one or two, the same way as they are redundant by having many leaves and flowers. But we now know that some but not all plants can somehow refill cavitated vessels with water. Presumably this occurs at night or during wet weather when the plant has extra water and there is no strong tension on the water columns. There is considerable research and some controversy about the mechanisms of cavitation, spread of embolisms, and vessel refilling. The repeated evolution of paratracheal parenchyma in angiosperm wood may be important.
SUMMARY
1. Living organisms transport materials over short distances (within organelles and cells) or over long distances (between nonadjacent cells).
2. Active transport is the forced pumping of material from regions where it is relatively unconcentrated to regions where it is more highly concentrated; active transport is an energy-consuming process.
3. Water potential (ψ) measures the capacity of the water to do work; in cells, it has two important components: osmotic potential (ψπ) and pressure potential (ψp). In soils and rather dry materials, a third component, matric potential (ψm), becomes important.
4. Water moves from regions where it is relatively concentrated (similar to pure water, ψ near zero) to regions where it is less concentrated (dissimilar to pure water, ψ more negative).
5. A cell’s water potential can be made more negative by pumping solutes such as K+ and sucrose into it or by depolymerizing polymers to monomers, especially starch to glucose. Reversing these processes increases a cell’s tendency to lose water.
6. A cell grows if its wall is too weak to counteract the tendency of water to enter the cell. Pressure potential cannot rise high enough to raise the cell’s water potential and bring the cell into equilibrium with its environment.
7. Incipient plasmolysis is the point at which the protoplast has lost just enough water that it no longer presses against the wall, and ψp equals zero. If the cell continues to lose water, it becomes plasmolyzed.
8. The water potentials of guard cells, motor cells, and sieve elements become more negative as these cells are forcibly loaded with solutes by active transport. Water flows into the cells, causing guard cells and motor cells to swell but the phloem sap to be squeezed out of sieve elements through sieve pores.
9. Water begins to flow from roots to shoots when the shoots lose water to the air and their water potential becomes negative enough to draw water from the roots and overcome the effects of gravity and friction.
10. As both air and soil become dry, tension on water columns in xylem increases; cohesion may be overcome and some water columns cavitate, forming embolisms.
11. Water’s adhesion to the sides of tracheary elements helps prevent embolisms; the presence of tracheids as opposed to vessels limits the amount of damage done if an embolism does form.
12. For non-CAM plants, light and low carbon dioxide stimulate stomata to open if moisture is adequate. Water stress overrides all other controls and causes stomata to close.
IMPORTANT TERMS
active transport
adhesive
apoplast
aquaporins
callose
cavitation
cohesion-tension hypothesis
cohesive
diffusion
embolism
eutrophication
incipient plasmolysis
long-distance transport
matric potential
megapascals (MPa)
molecular pumps
osmosis
osmotic potential
plasmolyzed
poikilohydry
P-protein
P-protein plug
pressure flow hypothesis
pressure potential
selectively permeable membranes
short-distance transport
sinks
sources
STM/CC complex
symplast
water potential
REVIEW QUESTIONS
1. Plants have both short-distance and long-distance transport. How long are the distances involved in each?
2. Plants have several tissues that act as isolation mechanisms. Name two.
3. Define diffusion. How does this differ from osmosis?
4. What is a freely permeable membrane? How does it differ from a completely impermeable membrane or a differentially permeable membrane?
5. Are the membranes in plant cells freely permeable, completely impermeable, or differentially permeable?
6. Would active transport be possible if the molecular pumps were located in a freely permeable membrane?
7. Like any other chemical, water has a free energy measured by its water potential. Name three simple ways water potential can be increased.
8. What are the three components of water potential? Which of these potentials measures water’s interaction with dissolved material?
9. The pressure potential of water measures the effect of pressure on water. If a cell had no cell wall, would it have a pressure potential?
10. Unless frozen, water is always in _______________, always ______________ within a plant from areas where it is abundant or under pressure to areas where it is rare or under tension.
11. Imagine that you have two solutions of glucose in water. One solution consists of 1 g of glucose in 100 mL of water. The other consists of 10 g of glucose in 100 mL. Which solution has a more negative osmotic potential?
12. In a beaker of pure water, what is the water potential? Does water potential become more positive or more negative as you add solute to it? Put pressure on it? Add it to dry clay? Add acid to it? In each case, which water potential component is changing?
13. By adding salt to eggplant, water can be drawn from the tissues. Which has a more negative water potential—eggplant or salt crystals?
14. What can you say about the water potentials of two solutions (or of a solution and a cell, or of two cells) when they are in equilibrium? At equilibrium, is there any net movement of water?
15. In each of the following pairs, circle the one that would probably have the more negative water potential: cell of a wilted leaf—cell of a turgid leaf; guard cells of an opening stoma—regular epidermal cells; guard cells of a closing stoma—regular epidermal cells; root cortex cell—moist soil; phloem cell being loaded with sucrose—leaf chlorenchyma cell; phloem cell unloading sucrose—tuber cell storing starch; clay with 10% moisture—silt with 10% moisture.
16. Imagine a cell with a water potential of —0.1 MPa being placed in a beaker of solution that also has a water potential of —0.1 MPa. Are the two water potentials in equilibrium? Would any water molecules be moving between the cell and the solution? Would there be a net movement of water? Now imagine a root in moist soil, and imagine that the root cortex cells have a water potential of —0.1 MPa and that the soil solution also has a water potential of —0.1 MPa. Would there be any net movement of water into the root?
17. Now imagine the same root as in Question 16 being placed in a dry soil in which the little soil water present has a water potential of —1.0 MPa. Would water move from the soil into the root or would it move from the root into the soil?
18. Circle the correct word of each pair.
a. If a cell [absorbs, loses] water, it will become turgid.
b. If a cell [absorbs, loses] water, it will become plasmolyzed.
c. If a cell [absorbs, loses] water and it has a very [weak, strong] wall, it will grow.
19. Plants never absorb so much water that their cells ______________, but they frequently lose enough water to ______________ because their protoplasts do not press firmly against the ________________ __________________.
20. All of the protoplasm of one plant can be considered to be one continuous mass, called the _______________________________. Walls and intercellular spaces of a plant are called the ___________________________ of the plant.
21. In glands, the apoplast consists mostly of __________________________. In nonglandular regions, the apoplast is mostly ________________________.
22. Are opening and closing of stomatal pores based on short-distance or long-distance transport? Is osmosis or active transport involved in opening and closing? What ion is especially important?
23. What is a motor cell? Name two plants that have them. Describe how motor cells adjust the position of a leaf.
24. In phloem transport, the sites from which water and nutrients are transported are known as __________ _______________________________ are sites that receive transported phloem sap.
25. During spring and summer, which organs are the dominant sources of sugar? When are tubers or fleshy taproots likely to be important sources?
26. As sugars are pumped into sieve elements, water follows. What happens? Do sieve elements merely become turgid?
27. Phloem sap is under pressure. What is the danger associated with this? How are P-protein and callose involved in counteracting this danger?
28. As sugar is actively transported into phloem in sources, what happens to the water potential of the cells losing the sugar? Does it become less negative?
29. Consider the pressure flow model of phloem transport. How do sugars and water enter the phloem from the source? How do sugars and water move from one phloem cell to another?
30. Can the direction of phloem transport change? Does phloem ever transport material into a leaf?
31. Water is both cohesive and adhesive. What do these words mean, and how do they affect water’s movement in a plant?
32. What is a leaf like early in the morning with respect to conditions that affect water movement? Describe what happens as the sun rises.
33. What is the speed of xylem sap translocation in ring porous trees?
34. In xylem, the pressure potential is a negative number. Why? What does the weight of the water have to do with this? Stolons, rhizomes, and horizontal vines do not have a problem with the weight of water. Why? In the epiphytic cactus Rhipsalis, the weight of water actually makes conduction easier. Why?
35. Many roots remain healthy with their water potentials as low as _____________.
36. Imagine lifting an icicle. You must pull against its _________________ and the __________________ of the icicle in a tube (the cell walls), but lifting the top of the icicle raises the ________________ icicle, unless it breaks.
37. Describe the cohesion-tension model of water movement through xylem. Would the weight of water be more of a problem in an upright tree or in a stolon? Why?
38. The breaking of a water column is called ________________. What breaks, the hydrogen bonds of the water or the cell walls of the tracheary elements?
39. When a water column cavitates, an air bubble is formed. The technical name is an _____________________.
40. In which habitat would you expect more cavitations—moist tropics or drier temperate areas? In which would you expect wood to have wide vessels, in which would you expect wood to have narrow vessels or just tracheids?
41. If a vessel cavitates and fills with air, can it ever be refilled with water?
Design Credits: Hummingbird: © Tongho58/Moment/Getty; Green plant cells: © ShutterStock, Inc./Nataliya Hora; Purple tulip: © ShutterStock, Inc./Marie C Fields; Dandelion: © ShutterStock, Inc./danielkreissl; Poppy: © ShutterStock, Inc./Saruri; Plant icon: © ShutterStock, Inc./Vector; Digging man icon: © ShutterStock, Inc./Z-art