Botany: An Introduction to Plant Biology - Mauseth, James D. 2017
Plant Physiology and Development
Soils and Mineral Nutrition

Chapter Opener Image: Plants, animals, soil, water, air (and microbes we cannot see), all are components of an elaborate life-sustaining network in which minerals, water, and energy move through Earth’s surface. Rock weathers to soil, roots absorb minerals and use them along with carbohydrates to build their bodies. Animals eat plants, capturing some of the minerals, energy, and organic compounds in their own tissues, passing others out of their bodies. At some point, microbes will consume the material, breaking down complex compounds and freeing the minerals such that roots can absorb them again. Rain may wash minerals into the water, which carries them far away, and wind blows dust from region to region. All nitrogen, which is essential to every form of life, ultimately comes from air. Canada geese (Branta canadensis).
OUTLINE
✵ Concepts
✵ Essential Elements
- Criteria for Essentiality
✵ Mineral Deficiency Diseases
- Causes of Deficiency Diseases
- Symptoms of Deficiency Diseases
- Mobile and Immobile Elements
✵ Soils and Mineral Availability
- Cation Exchange
- Soil Acidity
- The Endodermis and Selective Absorption of Substances
- Mycorrhizae and the Absorption of Phosphorus
✵ Nitrogen Metabolism
- Nitrogen Fixation
- Nitrogen Reduction
- Nitrogen Assimilation
- Other Aspects of Prokaryotes and Nitrogen
- Obtaining Nitrogen from Animals
✵ Storage of Minerals Within Plants
Box 13-1 Plants Do Things Differently: Plants Eat Dirt; Animals Eat Protoplasm
Box 13-2 Botany and Beyond: Acid Rain
Box 13-3 Plants and People: From Fertility Gods to Fertilizers
Box 13-4 Plants and People: Fertilizers, Pollution, and Limiting Factors
LEARNING OBJECTIVES
After reading this chapter, students will be able to:
✵ State three important functions of soil.
✵ Recall the three basic criteria for an element to be considered essential.
✵ Name several macronutrients and micronutrients.
✵ Identify the most significant (common) cause of soil mineral deficiency.
✵ Compare how mobile and immobile elements affect plant health.
✵ Explain how rock is converted to soil.
✵ Name the most important factor affecting soil acidity.
✵ Summarize the three components of the nitrogen metabolism process.
 Did You Know?
Did You Know?
✵ Plants contain only a small amount of minerals: The ash left after a plant or piece of wood is burned contains all its minerals.
✵ Fertilizers—either commercial or composted food scraps—are effective because of the minerals they make available.
✵ Commercial inorganic fertilizers can “burn” a plant if applied at a high concentration (dog urine is also too strong for many plants when “applied directly”).
✵ Sewage from cities, along with the runoff fertilizers from farms, overnourishes rivers and lakes, and usually causes excessive growth of algae and bacteria (called eutrophication).
![]() Concepts
Concepts
All organisms need elements such as nitrogen, phosphorus, calcium, magnesium, and sulfur. Plants must absorb these from soil and then use them and the glyceraldehyde-3-phosphate from chloroplasts to build all of their chemical components, however complex. This is an important concept: Plant metabolism is based on sunlight and chemicals present in water, air, and soil. No animal is able to survive on just minerals and one simple carbohydrate; they must obtain minerals and complex organic compounds in their food.
Most of the elements that are essential for plant growth and development are present in the crystal matrix of minerals. The elements become available to roots as rocks weather and break down, creating soil. During soil formation, rocks are converted gradually into dissolved ions and inorganic compounds. Because they are derived from the rock minerals, their role in plant nutrition is called mineral nutrition.
The term “mineral nutrition” covers a variety of types of plant metabolism. For some elements, after the mineral is absorbed from the soil, it can be used immediately as it is. An example is potassium, which is used by cells such as guard cells to adjust their turgor and water relations. Simple potassium ions are sufficient. Mineral elements such as iron and magnesium are more complex because they must be incorporated into compounds such as cytochromes or chlorophyll molecules before they are useful. Nitrogen is even more complicated: Like carbon, its oxidation state is important. Consequently, it must be reduced, and elaborate electron transport chains are necessary to convert it to useful forms.
The term “soil” covers a wide variety of substances. The various soils are important to plants not only in supplying minerals and harboring nitrogen-fixing bacteria, but also in holding water, supplying air to roots, and acting as a matrix that stabilizes plants, preventing them from blowing over. Critical aspects of soil are its chemical nature, which determines which mineral elements are present; its physical nature, which reflects its porosity, texture, and density; and its microflora and microfauna—the small animals, fungi, protists, and prokaryotes that live, respire, and gather food within the soil.
The microflora and microfauna of soil deserve special mention. It is easy to think of soil in terms of its chemical and physical properties only, but that would be an incomplete concept of soil. Most soils contain large amounts of microbes and tiny animals that are extremely important to plants (TABLE 13-1). Although microscopic to us, they are about the same size as roots and root hairs, and they interact extensively with root systems. For example, many soil microbes supply plants with nitrogen: Nitrogen is not found in rock matrixes, so soil formation does not make nitrogen available to plants. Instead, the primary source of nitrogen is molecular nitrogen (N2) of the atmosphere, but only certain bacteria and cyanobacteria have enzymes that convert molecular nitrogen into forms useful for metabolism. When these microbes die and decay, their organic nitrogen compounds are released to the soil and become available to plants.
TABLE 13-1 Number of Organisms in Soil
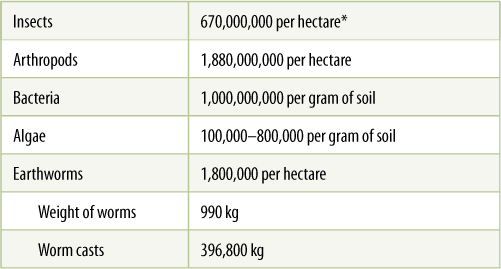
*1 hectare = 10,000 m2 = 2.45 acres. One American football field is 5,390 m2.
Just as the foods of animals vary, soils also vary in the quantities of minerals present, the texture of the soil, and the organisms present. Also, plants vary with regard to the amounts of minerals they require and their capacity for absorbing and processing minerals. All of these factors affect a plant’s health and, to a large extent, determine the types of plants that exist in a particular area.
![]() Essential Elements
Essential Elements
Much research in mineral nutrition involves experiments in which a single element is supplied to a plant in excessive quantities or withheld from it. This is accomplished by growing the plant in a hydroponic solution in which the chemical composition is carefully controlled. Hydroponic experiments were formalized and used extensively by Julius von Sachs in 1860. In such experiments, a solution is developed that supports plant growth. At first, this must be by trial and error, with numerous chemicals being added to the solution, and plants are tested to see whether they survive. Many tests are necessary because some of the chemicals may be toxic, even in low concentration. Other chemicals needed by plants are toxic if present at too high a concentration. Also, the form in which a chemical is present makes a difference: Nitrogen is important, but in addition to nitrate and ammonia, which plants use, other nitrogen compounds exist that plants do not use. Also, when numerous compounds are added to the same solution, unsuspected reactions may occur that create toxic compounds or convert useful compounds to useless ones.
After a solution of known composition is found which supports plant growth, an identical solution can be prepared except that one component is left out (FIGURE 13-1). If that component is not necessary for plant growth—that is, if it is not essential—plants grow normally, but if the excluded element is essential, the plant cannot grow correctly. For example, an experimental solution might contain nickel; if nickel is left out of the second solution, plants would grow well, perhaps even better than in the first solution; therefore, nickel is not an essential element. The second solution then becomes the main test solution. If a third solution is prepared similar to the second but lacking potassium, for example, no plant could survive; therefore, potassium is an essential element and must always be included (FIGURE 13-2). Using these hydroponic techniques, Sachs established a minimal nutrient solution that would support plant growth; it contained calcium nitrate, potassium nitrate, potassium phosphate, and magnesium sulfate. Currently, several solutions are known to support reasonable growth of most plants; two are Hoagland’s solution and Evan’s modified Shive’s solution. Many experiments begin with one of these.

FIGURE 13-1 In a hydroponics experiment such as this one at Penn State University, the test solution is often just a water solution in a bottle of boron-free glass. Air must be bubbled through the liquid to permit root respiration (clear plastic tubes). The bottles are wrapped in foil or painted black to exclude light, more closely resemble a soil environment, and prevent the growth of algae.

FIGURE 13-2 These plants are being grown in a hydroponic solution that contains all known essential elements except one—magnesium. Even though nitrogen, sulfur, and the other elements are abundant, they are of little use to plants if one essential element is missing. Growth and reproduction are governed by the least abundant factor, not the most abundant.
It may seem simpler just to grind up a plant and then extract and measure all of the chemical elements present, but plants actually absorb many elements they do not need—the endodermis simply cannot exclude them completely. A living plant usually contains at least trace quantities of every element present in the soil, whether essential to the plant or not.
The essential elements discovered by Sachs are called the major or macro essential elements because they are needed in large quantities by plants (TABLE 13-2). If dry plant material is analyzed, calcium, nitrogen, potassium, phosphorus, magnesium, and sulfur are present at concentrations of between 0.1% and 3.0% of the plant’s dry weight. Even as the first hydroponic experiments were being performed, botanists realized that the available chemicals were quite impure and that trace quantities of other elements were present. Despite their best efforts, they could not prepare solutions that had absolutely no copper, zinc, or many other elements. Thus, it was not possible to conclude that any particular element was completely nonessential; it was possible to determine only that relatively large amounts of most chemical elements were not essential.
TABLE 13-2 Elements Essential to Most Plants
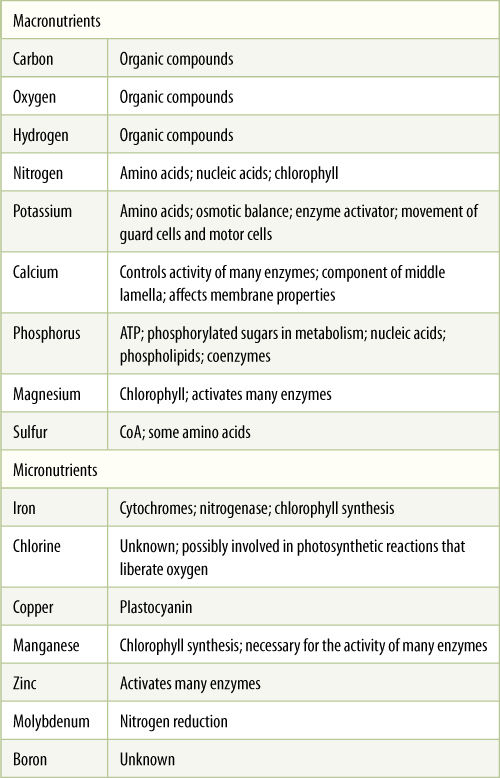
Except for boron, all these elements are also essential for us humans. But unlike plants, our diet must also provide us with fluorine, iodine, cobalt, selenium, chromium, and sodium. We obtain fluorine by adding it to our drinking water (fluoridation) and iodine is obtained by adding it to salt or by eating a large amount of seafood. Our lives depend on sodium and if we lose too much by sweating, we can quickly die. Most plants have no need for sodium at all; the exceptions are C4 plants and CAM plants, which need trace amounts.
As chemical methods improved, purer compounds were produced, and mineral nutrition experiments could be repeated with more certainty that the element tested had been almost completely excluded. With such improved chemicals, it was soon discovered that there exists a group of minor or micro essential elements, also called trace elements. Iron, boron, chlorine, copper, manganese, molybdenum, and zinc are required in extremely low concentrations by plants. Iron is the exception, being needed in amounts intermediate between those of the major and minor elements.
Although our reagents today are much purer than those of 100 years ago, it is still impossible to create a solution that contains absolutely only the nine major and seven minor elements. Our purest water has traces of many contaminants, the very best glass containers release silicon and boron into the solution, and all forms of chemical reagents have some trace contaminants. Chlorine was only recently discovered to be essential, at least for some plants; it remained undetected until it was realized that despite the purity of the test solutions, experimental plants receive adequate chlorine if scientists touch the plant because the skin of one fingertip has sufficient chlorine for an entire plant. Typical hydroponic experiments had shown that plants could grow in chlorine-free solutions, but when the studies were repeated with all experimenters wearing plastic gloves, the plants did not survive. Because such small quantities of chlorine are sufficient, we must suspect that other elements may also be necessary in similarly minute quantities; testing will continue whenever purer reagents become available.
Criteria for Essentiality
An element must meet three basic criteria to be considered essential.
1. The element must be necessary for complete, normal plant development through a full life cycle. If a particular element is required for any aspect of a plant’s growth, differentiation, reproduction, or survival, that element is essential. This logical and necessary criterion can be difficult to test. In a hydroponic experiment, the solution and plant must be carefully protected from contamination by dust and insects, both of which are mineral rich. Experiments must occur in laboratories, growth chambers, or specially controlled greenhouses. Laboratory conditions are not the same as the natural environment, however, and part of a plant’s ability to survive depends on its response to stress: cold, heat, drought, and pathogens. It may be that certain elements needed only under unusual conditions are not anticipated in a growth chamber experiment.
It is not feasible to study large trees in greenhouses or growth chambers, both because of their size and because it takes so many years to complete a life cycle. Because it is virtually impossible to test such species, the practical assumption is made that the major elements and most minor ones are essential for all plants, having been tested and found essential for many small herbs. This is a safe assumption if the role of the element is known. For example, nitrogen is present in amino acids and nucleic acids; because nothing can live without these, nitrogen is essential. The same is true for iron (cytochromes), calcium (middle lamella, enzyme control), and others. Chlorine is now known to be involved in the water-splitting reactions of photosynthesis, but the role of boron is still not known for certain, so we cannot assume with confidence that all plants require it. Furthermore, some desert plants have large amounts of silica in their epidermal cell walls; silicon is an essential element for them, but most plants appear not to need it at all.
2. The element itself must be necessary, and no substitute can be effective. This criterion is relatively straightforward; in most instances, if one essential element is absent, the presence of a chemically related element does not keep the plant alive. In some cases, elements can be substituted in specific enzymatic reactions or transport processes when studied in vitro, but even so, the entire plant cannot live with only the substitute. Some plants that require chlorine can survive if given large amounts of bromine, but such elevated levels of bromine do not occur in nature; therefore, the substitution works only in the laboratory.
3. The element must be acting within the plant, not outside it. The complexity of test solutions makes it difficult to analyze results in many cases. Iron is an essential element, but it is soluble in only a very limited range of acidity. Although iron may be added in adequate quantities, it often reacts with other chemical components and forms an insoluble precipitate. A test chemical can cause the precipitated iron compound to break down and form a soluble iron compound. With the new availability of iron, the plant grows well, even if the test element is not needed or absorbed. Without careful analysis, the test element would appear to be essential even though it is not used inside the plant.
This criterion has interesting ramifications. Most plants absorb phosphorus only poorly and depend on an interaction with soil fungi that absorb phosphorus and transfer it to the plant. If soil fungi are killed, plants grow poorly. If an element is essential to the fungus, is it then also essential to the plant even if the plant does not use it directly? This is one of those points that can be debated endlessly without being resolved; it is better to understand the biology than to dispute definitions.
![]() Mineral Deficiency Diseases
Mineral Deficiency Diseases
Causes of Deficiency Diseases
Virtually all types of soil contain at least small amounts of all essential elements; under natural conditions, it is rare to encounter plants whose growth and development are seriously disrupted by a scarcity or an excess of mineral elements. It is especially uncommon to find plants suffering from an overabundance of a particular mineral. In many cases, the unnecessary ions are not even absorbed by the roots. Certain types of excess minerals, if absorbed, can be precipitated in vacuoles as crystals; although a cell may contain large amounts of the mineral, only the ions actually in solution have any significant effect on metabolism.
Desert soils often have excessive amounts of all available minerals because ground water moves upward, carrying dissolved minerals with it. These can reach such strong concentrations that the water potential of the soil solution is extremely negative and roots are unable to extract water from it. Plants are unable to grow, not because of mineral toxicity but because of osmotic drought (FIGURE 13-3). Some species are adapted to less severely salty regions; saltbush (Atriplex) absorbs both water and salt but passes salt directly through the body and secretes it from salt glands located on leaves. This produces a coating of salt crystals that is thought to be selectively advantageous (FIGURE 13-4). The salt reflects away some of the excessive sunlight. It also makes the bush an unacceptable food because desert-dwelling animals must avoid salty foods in order to balance their salt/water intake.

FIGURE 13-3 As water evaporates from the soil, more water moves upward, carrying dissolved minerals that crystallize as the water evaporates. When soil water is present, its osmotic potential is extremely negative, as is its water potential (its pressure potential is zero). Roots cannot pull water out of such osmotically dry soil; they would die before their water potential became sufficiently negative. At the edge of a salt flat, salt concentrations are lower, and plants such as Atriplex (saltbush), Sueda (seepweed), and many grasses occur.
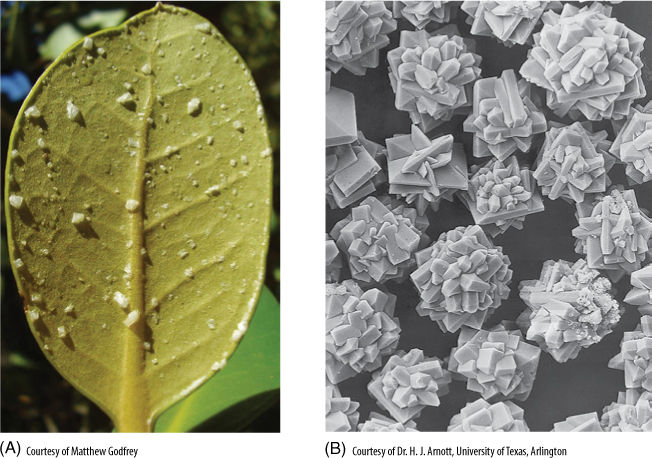
FIGURE 13-4 (A) Certain mangroves (Avicennia) of tidal marshes secrete salt through special glands. Manipulation and transport of such large amounts of salt require tremendous energy use, but salt excretion not only permits growth in saline habitats but also provides protection against herbivores. (B) Most plants neutralize excess salts by precipitating them as crystals such as these.
One of the most widespread examples of toxicity caused by elevated levels of single minerals is aluminum toxicity in acid soils. It is one of the main limitations on crop production on acid soils, which often occur in tropical areas with abundant rain and decaying plant matter. Mine tailings—the piles of discarded dirt extracted from mines after ore has been removed—often have such high levels of heavy metals that few or no plants grow on them.
Mineral-deficiency disease does not seem common in natural populations. Some soils may have such low concentrations of certain essential elements that some species are unable to thrive on them. For example, a type of soil called serpentine soil is extremely deficient in calcium, and few plants grow on it. Some species are more sensitive than others to low concentrations of essential elements (FIGURE 13-5). Because of competition with other plants and pathogens, the sensitive plants probably weaken and die early and do not reproduce successfully. Consequently, species especially sensitive to a particular deficiency typically do not occur in the plant community growing on soil deficient in that element.

FIGURE 13-5 Beans are especially sensitive to deficiency of zinc. On soils low in zinc, many plants grow well and are healthy but leaves of beans develop chlorosis and brown spots where the cells die.
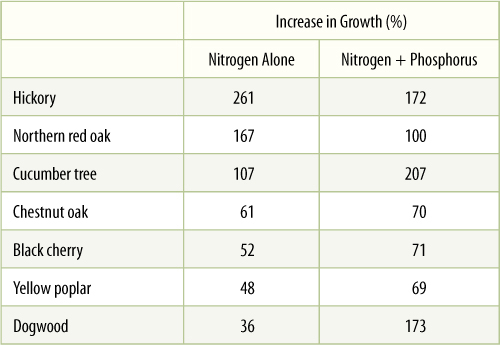
Deficiency diseases are most commonly encountered in nonnative crop plants or ornamentals. Crop plants especially have undergone human artificial selection (not natural selection) for traits such as rapid growth and high fruit/seed yield that require large amounts of nitrogen and mineral nutrients. Without fertilization, these plants often have poor growth and symptoms of deficiency diseases. As shown in TABLE 13-3, entire forests can be fertilized. The data show the results of fertilizing forests in Tennessee with 335 kg/hectare of fixed nitrogen, either with or without phosphorus. The addition of nitrogen was always beneficial, as virtually all soils are deficient in fixed nitrogen, but the addition of phosphorus often did not improve tree growth more than nitrogen alone. Soils naturally contain adequate phosphorus for many forest species.
The very act of harvesting crops leads to soil depletion: Fruits, seeds, tubers, and storage roots often have the greatest concentration of minerals in a plant. Harvesting them removes those minerals from the area; the rest of the plant body is relatively mineral poor and does not contribute much to re-enriching the soil even if it is plowed back in. With human populations, consumption of food may occur thousands of miles from the site of plant growth; even worse, waste products are dumped into rivers and carried away, never being returned to the soils. Under natural conditions, minerals are returned in the form of manure, which is typically deposited in the general region where feeding occurs. The most extensive crop removal is harvesting a forest for timber (TABLE 13-4), but several methods of harvesting are possible: removal of every part of the tree versus trimming and debarking, which permit the small branches, leaves, and bark to remain in the ecosystem.
TABLE 13-3 Fertilizing Forests
Symptoms of Deficiency Diseases
The particular symptoms of a mineral deficiency are more closely related to the particular element that is lacking than to the plant species; usually, all plants that suffer from a scarcity of a particular essential element show the same symptoms. One common symptom is chlorosis; leaves lack chlorophyll, tend to be yellowish, and are often brittle and papery. Deficiencies of either nitrogen or phosphorus cause another common symptom, the accumulation of anthocyanin pigments that give the leaves either a dark color or a purple hue. A lack of certain elements causes necrosis, the death of patches of tissue (necrosis can also be caused by bacterial, viral, and fungal infections). The location of the necrotic spots depends on the particular element: Potassium deficiency causes leaf tips and margins to die, whereas manganese deficiency causes the leaf tissues between veins to die even though all of the veins themselves remain alive and green.
Mobile and Immobile Elements
An important diagnostic aspect is whether symptoms appear in young leaves or older leaves. This is related to the mobility of the essential element. Boron, calcium, and iron are immobile elements; after they have been incorporated into plant tissue, they remain in place. They do not return to the phloem and cannot be moved to younger parts of the plant. A plant that grows in a soil deficient in boron, calcium, or iron is probably able to grow relatively well until the few available ions have been absorbed. Growth is normal until the soil is exhausted; further growth suffers mineral deficiency, and the newly formed tissues are affected (FIGURE 13-6A).
TABLE 13-4 Mineral Loss Through Crop Removal
Plants Do Things Differently
BOX 13-1 Plants Eat Dirt; Animals Eat Protoplasm
At its most fundamental level, plant nutrition is almost identical to that of animals, virtually indistinguishable. All cells depend on the same amino acids, nucleic acids, sugars, and with a few exceptions, the same lipids (plants never use cholesterol). Small molecules such as ATP and vitamins such as thiamin, riboflavin, and folic acid perform exactly the same functions in both types of organisms. At the same time, however, the two types of organisms could hardly differ more. No organism can synthesize mineral elements, of course, so plants and animals share that obvious similarity, but differences abound if we consider how an organism obtains organic molecules. Plants can be described first because they are so easy. They themselves make absolutely everything organic within their own bodies. It might be a bit difficult for zoologists, medical students, and dietitians to truly grasp this point. Every plant itself makes every organic molecule found within its body. A balanced diet for a plant is dirt, dirt, and more dirt, with carbon dioxide and water, morning, noon, and night. Photosynthesis converts carbon dioxide and water into glyceraldehyde-3-phosphate, and starting with just this simple small molecule and some minerals, a plant constructs everything it uses in its life—absolutely everything.
Animals lack many of these synthetic pathways and must obtain many organic compounds in their diet. We humans, like all other organisms, use a universal set of 20 amino acids in our proteins, but we cannot make 9 of these ourselves. We must obtain these 9 in the food we eat or we become ill and could even die. Several fatty acids cannot be synthesized by any tissue, cell, or organelle of our bodies. The list of essential nutrients is especially dramatic when it comes to vitamins, the organic molecules so fundamentally important in such small amounts that they were the first chemicals to be discovered as being essential dietary factors. Thirteen molecules have received this designation so far, and no one would be surprised if others are added to the list with further research. Every plant makes all of its own vitamins; we must get most of ours from our food. If an organic molecule is always reliably present in an animal’s food, then mutations that prevent the synthesis of the molecule are actually beneficial. The animal saves energy by not synthesizing compounds it will get in its diet anyway, and that energy can be used to carry out other life activities. If the vitamin is truly always available in the diet, then it is redundant for the animal to synthesize it as well.
The differences in nutritional resources used by plants versus animals are also great. Plants obtain nutrients in the form of elements or as simple compounds present in the environment, such as CO2, H2O, K+, Mg2+, SO42-, and so on. An animal begins with food in the mouth, but the nutrients occur as monomers in complex polymers, which in turn are parts of cell structure. Minerals must be digested away from organic molecules; for example, iron must be digested out of hemoglobin and myoglobin before it is absorbed into the blood stream. Although animals save energy by not needing to synthesize many molecules, they must go through much more effort to obtain their food and convert it to forms that can be absorbed. And their food usually also contains indigestible fur, feathers, bones, teeth, and dirt. Plants never take in such debris.
Plants are not completely self-sufficient nutritionally. Most rely on bacteria for converting atmospheric nitrogen gas (N2) into a chemical form such as nitrate (NO42-) or ammonium (NH4+) that plants and animals can use. Some plants have gone so far as to actually cultivate these bacteria within their own bodies, within nitrogen-fixing nodules on roots of alfalfa, for example, or within special chambers in liverworts. Although plants can take up phosphorus from the soil on their own, they usually obtain it more efficiently by entering into a symbiotic relationship—called a mycorrhizal association—with certain soil fungi that are more effective at scavenging phosphorus. Other plants have decided that animals have the right idea; the plants either are parasitic on other plants, or they capture and consume animals.
The elements chlorine, magnesium, nitrogen, phosphorus, potassium, and sulfur are mobile elements; even after they have been incorporated into a tissue, they can be translocated to younger tissue. After the soil becomes exhausted of one of these elements, older leaves are sacrificed by the plant. The mobile elements are salvaged and moved to growing regions (FIGURE 13-6B). The adaptive value of this is easy to understand: A leaf photosynthesizes most efficiently right after it has first expanded and less efficiently as it ages. The plant increases its overall photosynthetic rate by sacrificing old, inefficient leaves and using the minerals to construct new, efficient leaves.
The immobility of certain ions is not understood; boron, calcium, and iron are initially moved upward from roots into shoots, flowers, and fruits, so transport mechanisms do exist for them. Mutations that would result in the degradation of cytochromes in old leaves and the recovery of iron should be selectively advantageous. Animals have trouble with mineral recovery as well. For example, humans do not recycle the large quantities of iron in dead red blood cells; it is simply discarded, even if this results in anemia.

FIGURE 13-6 (A) This rose leaf is suffering from iron deficiency. Because the element is immobile, the little iron present in the plant cannot be transferred from older leaves to younger ones; therefore, young leaves show the disease symptoms. Cells near the veins have chlorophyll; those farther away are chlorotic. (B) These mature tomato leaves are suffering from a deficiency of phosphorus. Because the element is mobile, the plant can transfer atoms of phosphorus from older leaves to newer ones.
![]() Soils and Mineral Availability
Soils and Mineral Availability
Soils are derived from rock by processes of weathering. The initial rock may be volcanic (granite, basalt), metamorphosed (marble, slate), sedimentary (sandstone, limestone), or other types, but two things are important: Rock has a crystalline structure, and trapped within the structure are numerous types of contaminating ions and elements (FIGURE 13-7). Its crystal matrix prevents rock from being a suitable substrate for plant growth; it may contain most essential elements, but as long as they are part of the matrix or trapped by it, they cannot be absorbed and used by plants. Also, any water held by the rock as part of the crystal structure is unavailable to plants.
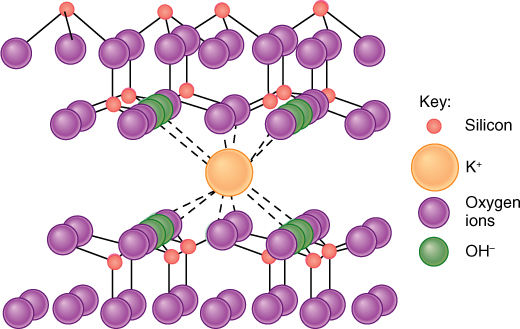
FIGURE 13-7 Rock is a complex, highly contaminated crystal; as it weathers, it gradually breaks down into soil. Within the crystal matrix of rock, numerous atoms of many different elements occur. As rock breaks apart, atoms at the surface are liberated into the soil solution. This is potassium trapped in vermiculite, a type of clay.
Two fundamental processes of weathering convert rock to soil: physical weathering and chemical weathering. As its name implies, physical weathering is the breakdown of rock by physical forces such as wind, water movement, and temperature changes. Ice is an important agent. During winter days, water from rain or melted snow seeps into capillary spaces within rock; at night, when temperatures fall below freezing, the expansion of water as it becomes ice causes cracks to widen. Portions of rock, ranging from small flakes to large pieces, are broken off. This is a slow process, but gradually, the average particle size of the rock is reduced.
Runoff from rainstorms, avalanches, and similar forces wash rock fragments and pebbles into streams and rivers, where physical weathering accelerates. In rapidly moving streams, the rocks are scoured by suspended sand grains; with high flow rates, rocks and boulders are carried downstream, grinding against each other and the stream bed. Wind-blown sand is a powerful erosive force, as are glaciers, which are extensive during the periodic ice ages.
Physical weathering produces a variety of sizes of soil particles; the largest ones that are technically important to soil are grains of coarse sand, with a size range of 2.0 to 0.2 mm. Particles only one tenth this large (0.2 to 0.02 mm) are fine sand, and those one tenth of this (0.02 to 0.002 mm) are silt. The finest particles, smaller than 0.002 mm in diameter, are clay particles, technically known as micelles (FIGURE 13-8).
The various particle sizes affect soil texture and porosity. In sands, particles are large and fit together poorly, so a great deal of space remains between particles. The spaces permit rapid gas diffusion, and roots in sandy soils typically are never starved for oxygen. During rain, the spaces fill with water, but typically, they cannot hold it against gravity because the spaces are too broad to act as capillary tubes. After rain stops, most of the water percolates downward and enters aquifers, flowing underground to wells, springs, and streams. Water that remains in the soil is held by capillary adhesion/cohesion (FIGURE 13-9) and is said to be the field capacity of the soil. Much of this water is available to roots.
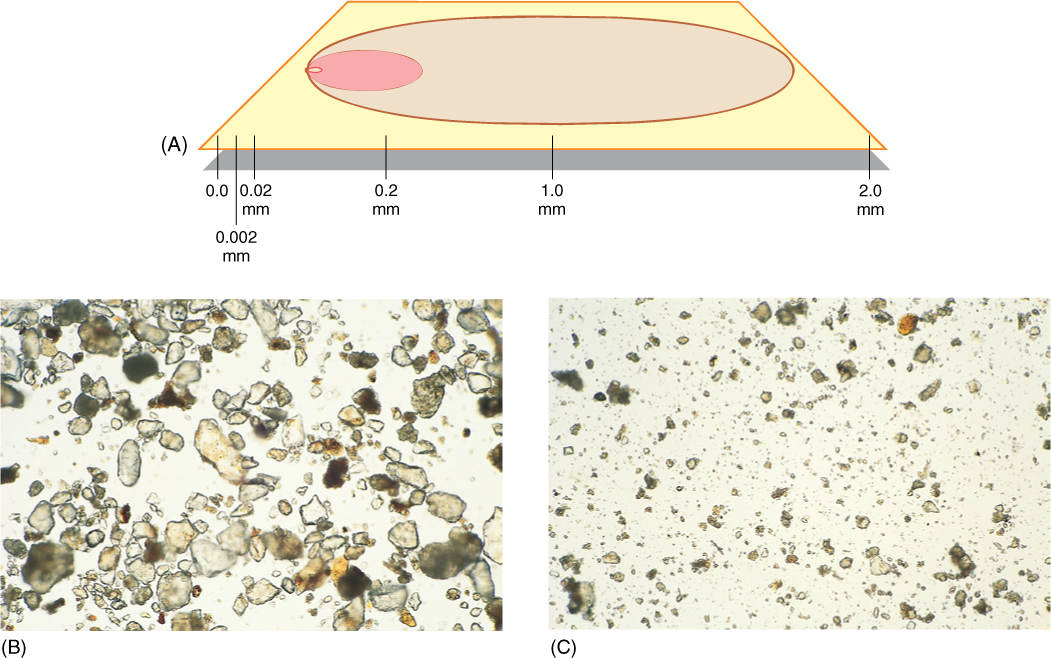
FIGURE 13-8 (A) Relative sizes of soil particles: From 0.2 to 2.0 mm is coarse sand; the next smaller is fine sand, and so on. It is easy to calculate the approximate amount of surface area, volume of rock, and volume of air/water space in a soil composed of only one type of particle. Assume you have 1 cm3 of pure coarse sand; the number of particles present is calculated by dividing 1 cm3 by the volume of a cube measuring 2.0 mm on each side (the volume of the particle plus its surrounding air/water space). Next, multiply the number of particles by the volume of a sphere with a radius of 1.0 mm (each sphere has a diameter of 2.0 mm). This gives the total volume of rock in 1 cm3 of soil. Subtract this from 1 cm3 to obtain the volume of air or water that can be held by the soil. Finally, calculate the surface area of each sphere and multiply that by the number of particles to obtain the total surface area that is releasing minerals into the 1 cm3 of soil. Now do this for fine sand, silt, and clay. Which soil has the most surface area? The greatest amount of air and water? (B) Particles of fine sand, viewed by light microscopy (× 60). (C) Particles of silt and micelles of clay (× 60).
Chemical weathering involves chemical reactions, and the most important agents are acids produced by decaying bodies, especially those of plants and fungi. In addition, many organisms secrete acids while alive, and the carbon dioxide produced during respiration can combine with water, forming carbonic acid. When an acid dissolves in water, it dissociates into a proton and an anion, both of which interact chemically with rock’s crystal matrix and with embedded contaminant elements. In regions with a great deal of warmth, moisture, and abundant decaying vegetation, such as tropical regions, chemical weathering can be extremely rapid, and thick soils accumulate in a short time. In drier regions with long, cold winters and less vegetation, such as temperate mountains and the prairies of the plains states and Canada, fewer acids are available and chemical weathering is slower. It may take as long as 10 million years for just 1 cm of soil to form. Chemical weathering is greatly increased if rock has already been reduced to sands and silts by physical weathering; these have a large surface area for the acids to attack.
Chemical weathering decreases soil particle size, but more importantly, it alters soil chemistry. As the crystal matrix dissolves, matrix elements become available to the plant, and trapped elements are liberated. As the matrix breaks down, positively charged cations are freed; thus, the residual undissolved matrix has a negative charge (FIGURE 13-10). With coarse particles such as sand and silt, the surface-to-volume ratio is so small that the charge is not important, but with clay micelles, the total amount of surface per unit of soil volume is great. Because the particles have a negative charge, cations such as K+, Ca2+, Mg2+, and Cu2- are held near the particles’ surfaces. The bonding is much weaker than that of the crystal matrix, therefore, roots can absorb the cations. This attraction to the micelle surface is beneficial; without it, many important cations would be washed deep into the soil by passing rainwater.
Cation Exchange
Because cations are loosely bound to micelle surfaces due to their charge, roots cannot absorb them directly. Instead, the cations must first be freely dissolved in the soil solution; this is done by cation exchange. Roots and root hairs respire, giving off carbon dioxide. As this dissolves in the soil solution, some reacts chemically with water, forming carbonic acid, H2CO3. This breaks down into a proton and a bicarbonate ion (FIGURE 13-11), which can further dissociate into a second proton and a carbonate ion. The presence of protons acidifies the soil solution adjacent to roots and root hairs; as the protons diffuse, they bounce close to a bound cation at a micelle surface. The presence of the proton’s positive charge disrupts the electrical attraction of the cation, liberating it and trapping the proton. Because the proton was derived from waste carbon dioxide, its loss does not hurt the plant. The liberated cation may diffuse in the direction of the root and be absorbed and transported upward by xylem, or it may diffuse away from the root or strike another micelle, liberating either a proton or another cation. Over time, however, large quantities of cations are absorbed. Acidity caused by secretion of acids by bacteria and fungi, by the decomposition of humus, and by acid rain also result in the liberation of cations.

FIGURE 13-9 (A) This sandy soil has such large spaces between its particles that it cannot hold water well; it dries quickly after a rain. It has a low field capacity. (B) As water is pulled away from soil by gravity, it percolates downslope into valleys. Soil at the bottom of ravines has water available longer than soil on the sides of a slope; consequently, more vegetation grows at the bottom of a valley than on the sides. (C) This field has been out of production for a year; no crop is cultivated on it, and we say that it is a “fallow” field. Being fallow helps the soil in many ways. Soil particles break down a bit and release more nutrient ions; the straw from last year’s crop decomposes and releases nutrients that had been bound in the walls and protoplasm. Many disease organisms die if there are no living plants for them to attack.
Soil particles are not the only structures that hold cations. Decaying organic matter also forms negatively charged matrixes. Cellulose crystals of cell walls in mulch and humus are especially valuable not only for holding cations liberated from rock weathering but also for retaining the essential elements released by decaying protoplasm. Such organic matter also holds water and greatly improves the quality of any type of soil.
Soil Acidity
Soil pH, the concentration of free protons in the soil solution, is important for cation exchange and the retention of cations in the soil during heavy rain. As acidity increases (pH becomes lower), the greater concentration of protons causes more cations to be released from soil micelles; these may be absorbed by roots or washed away in ground water. An extremely acid soil (pH of 4.0 to 5.0) tends to lose cations too rapidly and becomes a relatively poor soil. On the other hand, highly alkaline soils (pH of 9.0 to 10.0), which are frequent in dry climates, have too few protons to allow cation release, and concentrations of minerals can become excessively high.
FIGURE 13-10 As rock weathers, the negatively charged components are most resistant, so the rock fragments have a negative surface charge. Because of this, the cations released by weathering do not completely leave the rock, but are held by very weak electrical attraction.
Soil pH affects the chemical form of certain elements, causing them to change solubility. In acidic soils, aluminum and manganese can become so soluble as to reach toxic levels. In alkaline soils, iron and zinc become quite insoluble and unavailable to plants, but molybdenum is more soluble at a high pH. In general, a pH between 6.5 and 7.0 is best for many elements, especially iron, zinc, and phosphorus.
Many factors affect soil acidity, such as the chemical nature of the original rock, but probably the most important factor is rainfall. With high rainfall, there tends to be an abundance of vegetation that produces acids by means of respiration, excretion, and decay. With low rainfall, not only is there little vegetation, but there may not be any washing out of the soil. Cations build up, increasing soil alkalinity by increasing the concentration of hydroxyl ions. Just as dissociation of an acid produces a proton and an anion, dissociation of a base produces a hydroxyl and a cation:
NaOH → Na+ + OH-
Because some soils are more acidic than others, plants have adapted to differences in the availability of essential elements (TABLE 13-5). Natural selection favors mutations that allow desert plants to cope with alkaline soils, whereas plants of wet areas must become adapted to acid soils. For example, azaleas, camellias, gardenias, and rhododendrons absolutely must have acidic soil; if planted into alkaline soils, they show stress symptoms immediately, often suffering from lack of iron as well as general poor health (FIGURE 13-12). Alfalfa, apples, broccoli, and hydrangeas require an alkaline soil, whereas most plants do best if the soil pH is near 6.0 to 6.5. Some plants are able to tolerate a wide range of soil acidity, but typically, plants show best health and vigor only in soil with the optimal pH.
TABLE 13-5 Species Adapted to Acidic, Neutral, and Alkaline Soils
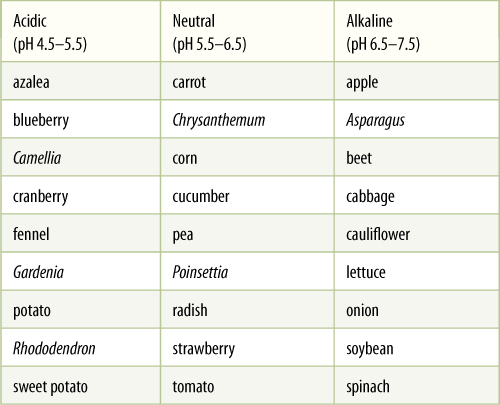
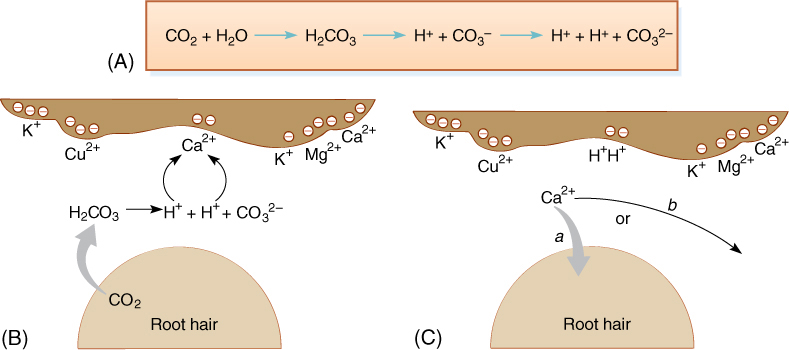
FIGURE 13-11 (A) The reaction of water and carbon dioxide results in carbonic acid (H2CO3), most of which dissociates into a proton and a bicarbonate anion. Some of the bicarbonate dissociates further, releasing another proton and a carbonate anion. (B) Protons from carbonic acid may diffuse close enough to a cation to disrupt its attraction to a soil micelle, liberating it. (C) As the cation then diffuses through the soil, it may encounter a root (arrow a) or it may not (arrow b).
Botany and Beyond
BOX 13-2 Acid Rain
Acid rain is a silent killer, destroying forests, streams, and lakes throughout the world. As we burn fuels rich in sulfur, such as much of the coal used to generate electricity, the sulfur burns to sulfur dioxide and is emitted through the smokestack of the generating plant. In the air, sulfur dioxide reacts with water to form sulfuric acid, which then dissolves into the water droplets of clouds. As the drops fall as rain, snow, or sleet, they carry the sulfuric acid with them as acid rain, also called acid precipitation. Sulfur dioxide gas itself enters stomata and is converted to sulfuric acid inside the leaves as it dissolves into wet cell walls and cytoplasm.
Acid precipitation damages plants in many ways. Because the cuticle is not absolutely impermeable, some acid slowly moves directly into the plant tissues and damages leaves, flowers, fruits, and cones. Perhaps more significantly, most of the acid enters the soil and accelerates cation exchange, causing positively charged ions to be released from the soil particles and to be washed away. Soil is left depleted of nutrients, and plants suffer from mineral deficiency. Downwind of the most heavily polluting industrial centers of Germany, entire forests are dying or are dead. Pollution from the United States and Canada is causing extensive damage to North American forests.
As acid rain accelerates cation exchange, minerals are washed from the soil and enter streams, in effect fertilizing them and causing rapid growth of algae. In small quantities, this provides more food for fish, turtles, and other aquatic animals, but in many cases, the algal growth is so abundant that it forms a massive, impenetrable layer across the top of a lake or slowly moving river. As the algae die, their bodies sink and are attacked by decomposers, mostly bacteria. Bacterial decomposition consumes oxygen, and before long, the bottom of a lake or quiet river is an anaerobic dead zone with too little oxygen to support any animal life. This process is called eutrophication, and the result is a lake or river that is basically dead, having little life other than a mat of algae at its surface.

FIGURE B13-2 These conifers have been damaged by acid rain. The acid rain does not always destroy a forest but instead may alter the species composition and diversity.
The Endodermis and Selective Absorption of Substances
Elements in the soil solution, whether essential or not, can enter roots either by crossing a plasma membrane and entering the symplastic protoplasm phase of the plant or by diffusing along cell walls and intercellular spaces in the apoplastic phase. In the first method, the selective permeability of the plasma membrane and the presence or absence of molecular pumps control entry of ions and molecules—certain substances can be excluded and others can be actively transported in; however, a substance can penetrate the root epidermis and cortex simply by moving through the water in cell walls and intercellular spaces. No metabolic control exists, and even harmful substances enter. If all substances could enter the xylem transpiration stream, they would have access to all cells of the plant body.

FIGURE 13-12 This azalea leaf is from a plant growing in alkaline soil. Azaleas require acid soils and suffer iron deficiency in alkaline soils.
The endodermis prevents uncontrolled, apoplastic diffusion in roots. The Casparian strips on all radial walls are impermeable to water and water-borne solutes; nothing can cross it simply by diffusion (FIGURE 13-13). For a substance to penetrate beyond the root cortex, it must first enter the endodermal cell protoplasm by being accepted across the plasma membrane of a cell in the root epidermis, cortex, or endodermis. Its highly selective permeability allows the endodermis to control which elements enter the transpiration stream.
Mycorrhizae and the Absorption of Phosphorus
The roots of 90% of all species of plants form a symbiotic association with soil fungi, and this relationship is called a mycorrhiza; the symbiosis permits plants to absorb phosphorus efficiently. In the most common type, vesicular/arbuscular mycorrhizae, some fungal filaments penetrate root cortex cells and then branch profusely, forming a small tree-shaped arbuscule inside the cell; other filaments swell into balloon-like vesicles. The fungus collects phosphorus from the soil and transports it into arbuscules, where it accumulates as granules. After the arbuscules fill with phosphorus, the granules gradually disappear as phosphorus is transported into the root cell protoplasm. After the transfer is complete, the arbuscule collapses and the root cell returns to normal. This mycorrhizal symbiosis is essential to most plants; plants in sterilized soil grow poorly and show signs of phosphorus deficiency even if the soil contains adequate amounts of phosphorus. In soils with very high levels of available phosphorus, mycorrhizae may be less important.
![]() Nitrogen Metabolism
Nitrogen Metabolism
Nitrogen does not occur as a component of rock matrixes nor as a contaminant in rock; the most abundant source of nitrogen is the atmospheric gas N2. This nitrogen is relatively inert chemically and is useless to almost all organisms; it must be converted to chemically active forms. This process, called nitrogen metabolism, consists of (1) nitrogen fixation, (2) nitrogen reduction, and (3) nitrogen assimilation.
Nitrogen Fixation
Nitrogen fixation is the conversion of N2 gas into nitrate, nitrite, or ammonium, all forms of nitrogen that are substrates for a variety of enzymes. One means of nitrogen fixation is human manufacturing; the fertilizer industry synthesizes either nitrate or ammonium from atmospheric nitrogen, but this is an extremely expensive and energy-intensive process. About 110 million tons of nitrogen fertilizer are produced annually. Over half of all the energy used in agriculture is consumed in the production, distribution, and application of nitrogen fertilizers.
Natural processes fix over 190 million tons of nitrogen annually. Lightning is important; the energy of a lightning strike passing through air converts elemental nitrogen to a useful form that dissolves in rain and falls to the earth. However, nitrogen-fixing bacteria and cyanobacteria are by far the most important means of fixing atmospheric nitrogen, annually converting 130 million tons of nitrogen to forms that plants and animals can use. These organisms have nitrogenase, an enzyme that uses N2 as a substrate. It forces electrons and protons onto nitrogen, reducing it from the +0 to the —3 oxidation state (TABLE 13-6). Ammonia, NH3, is the product; it immediately dissolves in the cell’s water and picks up a proton, becoming the ammonium ion, NH4+ (ammonia is the nonionized form and ammonium is the dissolved, ionized form; in both, nitrogen is in the —3 oxidation state). Nitrogenase is a giant enzyme complex composed of two distinct enzymes (dinitrogenase composed of four proteins, and dinitrogenase reductase composed of two proteins); it has a molecular weight of 300,000 Daltons and contains numerous atoms of iron, molybdenum, and sometimes vanadium (depending on the species). It is extremely sensitive to oxygen and functions only if oxygen is completely excluded from it. Like RuBP carboxylase, nitrogenase is an extremely slow enzyme, binding and reducing as few as five molecules of N2 per second.
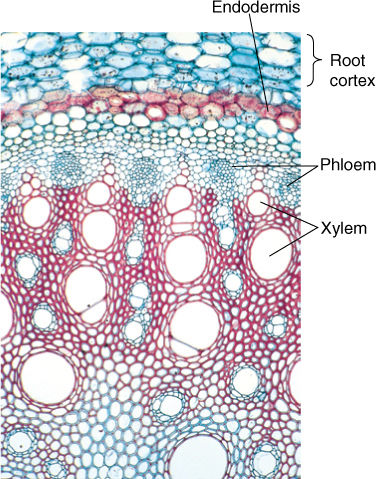
FIGURE 13-13 The endodermis in roots prevents uncontrolled diffusion of minerals into the xylem (× 50).
TABLE 13-6 Oxidation States of Nitrogen Compounds
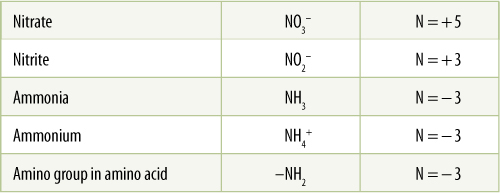
Like carbon, nitrogen can hold various numbers of electrons in more or less stable bonding orbitals. The partial charge on nitrogen can be calculated by adding the partial charges on protons (+ 1) and oxygens (− 2). Reducing nitrate to nitrite requires two electrons; reducing nitrite to ammonia requires six.
Some nitrogen-fixing microorganisms are free living in the soil; examples are Azotobacter, Clostridium, Klebsiella, and Nostoc (FIGURE 13-14). The nitrogen they fix is used in their own metabolism and becomes available to plants and fungi only when they die and their bodies decay. Other nitrogen-fixing organisms live symbiotically, growing inside tissues of host ferns and seed plants (TABLE 13-7, FIGURE 13-15). The best known examples are root nodules on legumes such as alfalfa; the nodules are growths of root tissue whose cells contain bacteria of the genus Rhizobium. Plants such as alders (Alnus), bog myrtle (Myrica gale), and Casuarina equisetifolia are pioneer plants that are the first to grow in poor, nitrogen-deficient soils such as bogs, sand dunes, and glacial rubble. These species obtain their nitrogen from a symbiosis with the prokaryote Frankia. The symbiotic bacterial cells use part of the fixed nitrogen for their own growth and reproduction, but they also permit large amounts to leak out into the protoplasm of the surrounding root cells. Symbiotic nitrogen fixers usually produce fixed nitrogen at a much greater rate than free-living microorganisms, perhaps because they have the energy resources of the plant at their disposal.
The rate at which plant/prokaryote symbioses fix nitrogen is strongly influenced by the stage of development of the plant: When soybeans begin to produce their protein-rich seeds (40% protein, the richest seeds known), nitrogen fixation in the roots increases greatly. As much as 90% of all nitrogen fixation occurs during the phase of seed development, whereas only 10% occurs during all the vegetative growth that precedes it. Furthermore, if legume crop plants are given high levels of nitrogen fertilizer, plants that have not yet formed nodules do not produce them, and those that already have bacteroid-filled nodules decrease the amount of nitrogen fixed and even allow the nodules to senesce. With adequate nitrogen available in the environment, it is selectively advantageous not to pass glucose on to bacteria.
Nitrogen Reduction
Nitrogen reduction is the process of reducing nitrogen in the nitrate ion, NO3-, from an oxidation state of +5 to the —3 oxidation state of ammonium, which is also the oxidation state of nitrogen in amino acids, nucleic acids, and many other biological compounds (see Table 13-6). Nitrogenase automatically reduces nitrogen during the fixation process, and if a plant can absorb that form of nitrogen, no further reduction is necessary. Also, as organic matter decays in the soil, ammonium is released and becomes available. Unfortunately, for plants, ammonium is an extremely energetic compound that numerous species of soil bacteria use as “food,” oxidizing it to produce ATP. In the process, ammonium is converted to nitrate. Such soil bacteria are so common that ammonium lasts only a short time in soil, and the predominant form of nitrogen available to roots is nitrate. Due to competition from soil microbes and leaching during rain, crop plants take up only about half of all artificial fertilizers applied to fields.
Reducing nitrate back to ammonium requires eight electrons for each nitrogen atom and a great deal of energy. In the first step, two electrons are added, reducing nitrogen from +5 to +3 and forming nitrite, NO2-. The enzyme is nitrate reductase, and it carries electrons by means of a molybdenum atom (FIGURE 13-16). Just like electron carriers in photosynthesis or respiration, when nitrate reductase reduces nitrate, it becomes oxidized and must pick up more electrons. It gets these from reduced flavin adenine dinucleotide (FADH2) and reduced nicotinamide adenine dinucleotide (NADH); the ultimate source of energy and electrons is respiration.

FIGURE 13-14 Cyanobacteria are common components of most soils, although usually they are quite inconspicuous. These are colonies of the cyanobacterium Nostoc. The colony swells and fixes nitrogen rapidly when wet (A), but it becomes dormant and crisp when dry (B). Even though extremely desiccated with an extraordinarily negative water potential, the cells are alive and revive within seconds of receiving water. (C) The largest of these Nostoc cells are heterocysts, which carry out nitrogen fixation (× 300).
TABLE 13-7 Plants That Form Associations with Nitrogen-Fixing Prokaryotes
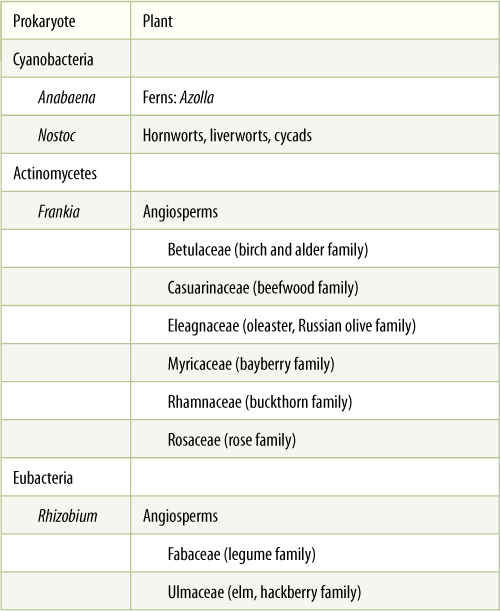
In the second step, nitrite reductase adds six electrons to nitrite, reducing it to ammonium. The process is not well understood, but extremely strong reducing agents are needed. Apparently, nitrite reduction in leaves is powered by reduced ferredoxin from the light reactions of chloroplasts; however, roots are a more important site of nitrite reduction, and of course, they have no light reactions. Instead, they use reduced nicotinamide adenine dinucleotide phosphate (NADPH) produced by the pentose phosphate pathway. Although ATP is not consumed during nitrogen reduction, the process is expensive energetically because the NADH, NADPH, and ferredoxin used are no longer available for ATP synthesis in the mitochondrial electron transport chain.

FIGURE 13-15 These plants of alfalfa (Medicago sativa) will be used to feed cattle, horses, and other livestock. Like many species in the legume family (Fabaceae), this has root nodules that contain nitrogen-fixing Rhizobium bacteria. Consequently, alfalfa plants can grow well in poor soil and produce protein-rich leaves, which are especially nutritious as animal feed.
In leaves, nitrate and nitrite are present as a result of breakdown of amino acids, nucleic acids, and other nitrogenous compounds during normal metabolism; nitrogen reduction recycles the nitrogen and conserves it within the plant.
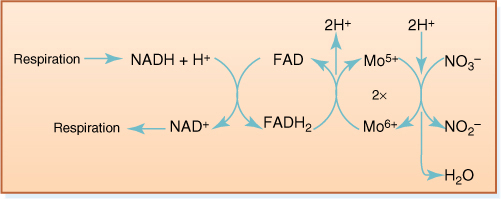
FIGURE 13-16 The electrons that reduce nitrate to nitrite are brought to it by a short electron transport chain. FAD and molybdenum are actually bound to the nitrate reductase enzyme, but NADH and NAD+ diffuse between the enzyme and sites of respiration.
Nitrogen Assimilation
Nitrogen assimilation is the actual incorporation of ammonium into organic molecules in the plant body. The process is similar to that of an electron transport chain: Reduced nitrogen passes through a series of carriers that function repeatedly but in the long run are not changed.
The acceptor molecule is glutamate (the ionized form of glutamic acid); it reacts with ammonium and ATP, producing glutamine and ADP (FIGURES 13-17 and 13-18). Glutamine transfers the ammonium, now referred to as an amino group, to α-ketoglutarate, transforming both molecules into glutamate. The original acceptor has been regenerated, and an extra molecule of glutamate has been produced which can in turn transfer its amino group to another molecule. If the next molecule to receive the amino group is oxaloacetate, the amino acid aspartate is produced. If pyruvate receives the amino group, the amino acid alanine is produced.
These glutamate-mediated transfers of amino groups are the basis for incorporating nitrogen into the plant’s metabolism and synthesizing all of the amino acids, nucleotides, chlorophyll, and many more compounds. The transfer of an amino group from one molecule to another is transamination.
Nitrogen assimilation usually occurs in roots, the site of either absorption of nitrate or ammonium or transfer of ammonium from symbiotic prokaryotes. Much of the assimilated nitrogen must be transported to the shoot through the vascular tissues. Several nitrogen-rich compounds are common: Asparagine and glutamine both contain two amino groups rather than just one, as in the corresponding amino acids (Figure 13-18). In legumes, allantoic acid and allantoin are common transport forms, and in alders, nitrogen is carried from roots as citrulline.
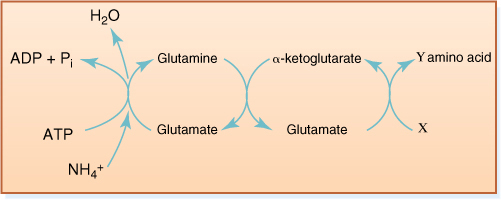
FIGURE 13-17 Nitrogen assimilation occurs by means of an “amino transport chain.” The acceptor molecule is glutamate, which is already an amino acid, having one amino group. When it picks up ammonia, it becomes glutamine, an amine, having two amino groups. One is passed on to α-ketoglutarate, regenerating the acceptor and producing a new carrier. The next step is variable: By using various acids at X, the cell produces different amino acids at Y. If oxaloacetate is used, the amino acid aspartate results.
Other Aspects of Prokaryotes and Nitrogen
Whereas certain bacteria reduce and fix nitrogen, others oxidize it, which adversely affects plants. When organisms die and decay, their nitrogenous compounds become available to the roots of living plants; however, because these compounds are highly reduced, they can be used as an energy source if oxygen is available. Certain soil bacteria are nitrifying bacteria: They oxidize ammonium to nitrite (Nitrosomonas, Nitrosococcus), and others oxidize nitrite to nitrate (Nitrobacter, Nitrococcus). The entire process is called nitrification. Both types of nitrifying bacteria are so common that nitrite never builds up in the soil. Nitrification has important ecological consequences; although plants can absorb and use nitrate, it is more readily washed from soil because it is negatively charged and remains in solution, whereas ammonium is positively charged and is bound to soil particles. Also, when ammonium is absorbed by a plant, the nitrogen is reduced already; however, when nitrate is absorbed, large amounts of ATP must be used to reduce it before it can be incorporated into amino acids, nucleotides, and so on.
Denitrification is a process in which certain bacteria (Hyphomicrobium, Pseudomonas) reduce nitrate to gaseous nitrogen, N2. Nitrate or nitrite is used as an electron acceptor during energy production. Whereas nitrification results in nitrate that plants cannot use as easily as ammonium, denitrification results in nitrogen gas, which plants cannot use at all.
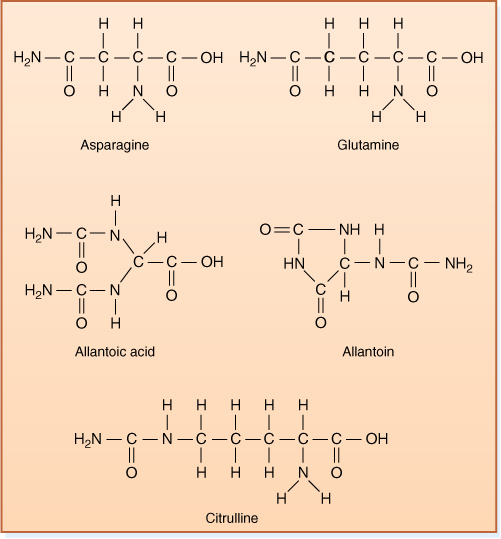
FIGURE 13-18 These chemicals each have a high nitrogen-to-carbon ratio and are transport forms of reduced nitrogen. Once moved through the phloem to a nitrogen sink, the compounds are catabolized and the amino group is used in the synthesis of amino acids, nucleic acids, and other compounds.
Obtaining Nitrogen from Animals
Soils in bogs and swamps typically have very little nitrogen available because of nitrifying and denitrifying bacteria. Many bog-adapted plants obtain a significant fraction of their reduced nitrogen by catching animals; these are carnivorous plants. Carnivory has originated many times, in many different groups of plants; thus, there are numerous mechanisms that trap and digest animals. It is important to point out that trap leaves photosynthesize as well as catch, digest, and absorb insects. Carnivorous plants obtain their energy through photosynthesis, not from the fats, carbohydrates, or proteins of the animals they consume.
Ant-plants are flowering plants and ferns that also obtain reduced nitrogen from animals; examples are Myrmecodia, Hydnophytum, and Solanopteris (FIGURES 13-19 and 13-20). They are epiphytic, with their roots attached to tree trunks or branches, and bark is a nitrogen-poor “soil.” Ant-plant stems are swollen and have hollow chambers that ants use as living spaces, as bathrooms, and as graveyards for ant corpses. As wastes and dead ants decompose, ant-plants absorb nitrogenous compounds either by means of absorptive areas on the chamber walls themselves or by means of adventitious roots that grow into the waste-filled chambers. Rather than trapping and harming the ants, the plants provide living space; therefore, this relationship is mutually beneficial and is said to be mutualistic. Any chamber formed by a plant and used commonly as a living space by an animal is a domatium (pronounced doe MAY shum; plural domatia).
![]() Storage of Minerals Within Plants
Storage of Minerals Within Plants
Neither plants nor animals can be certain that essential minerals will be available in appropriate quantities throughout their lives, so storage mechanisms are necessary. Animals tend to have large reservoirs of at least some elements: The copious amounts of protein in muscle can be broken down to amino acids that then donate their amino groups to supply nitrogen. Bones and teeth store both calcium and phosphate. For some reason, plants rarely store minerals or nitrogen as crystallized or polymerized forms the way animals do, even though a plant’s mineral requirements change greatly. During formation of flower buds and then especially during maturation of fruits and seeds, the need for nitrogen, phosphorus, potassium, and other essential elements increases greatly, whereas wood synthesis may require huge amounts of carbohydrate but almost no mineral resources. Trees such as cherry, which blossom in early spring and then produce both fruits and leaves at the same time, have a tremendous demand for essential elements early in the year but require very little during summer.
Plants and People
BOX 13-3 From Fertility Gods to Fertilizers
The field of mineral nutrition is excellent for studying the development of biological thought. It is easy to assume that people have always understood the importance of nitrogen, potassium, phosphorus, and other elements, even if they did not study it explicitly. After all, humans have been farming for thousands of years, but the way we think of things now is different from other approaches to understanding the world. The first agricultural societies, those of Sumer, Egypt, India, and China, thought in terms of fertility gods; plants were believed to grow well or poorly according to the whims of divine intervention.
The earliest attempts to understand plant growth in a nonmystical way were formulated by ancient Greeks. Their thoughts are usually summarized by the phrase “Plants eat dirt.” This is not as simplistic as it sounds. The Greeks believed that the universe contained only four “elements”: earth, water, fire, and air. All things, plants and animals included, were constituted of various combinations of those elements. In such a world, the idea of plants as transmuted earth makes sense. Plants do not grow unless their roots are in earth, they must have water, and of course, they can burn, so the element fire must be contained as well.
This concept remained basically unchanged until the 1600s. By then, it was known that there are many elements, although the concept of “element” was not perfectly clear. The periodic chart had not been developed yet by Mendeleev, and the belief that lead could be changed into gold was still held. In 1644, J. B. van Helmont published the results of an important experiment. He had made a cutting of a willow and allowed it to form roots. When it weighed 5 pounds, he planted it in a container holding exactly 200 pounds of soil. He watered the willow and allowed it to grow for 5 years, then removed it and cleaned off the roots, being careful not to lose any soil. The plant had increased in weight to 169 pounds, but the soil had lost almost nothing—it still weighed 199 pounds, 14 ounces. Clearly the plant was not composed primarily of transformed earth; van Helmont concluded that water was the important transformed element (carbon dioxide was not yet known to exist).
This was a tremendous advance for two reasons. If water could be transmuted to vegetable matter, then it must be a compound and not an element. The second reason lay in the concept of experimentation. Greek science had been based on observations combined with thought, reasoning, and logic, but without experimentation. Van Helmont’s results showed not only that the Greek conclusion was incorrect, but that their method of study without experimentation was inadequate.
Van Helmont’s work was followed by that of John Woodward, who tested various types of water without soil: rainwater, stream water, and water from mud puddles. He found that rainwater was least effective in promoting plant growth, even though it was known to be the purest type of water. Chemical methods still were not advanced enough for him to be able to analyze why water from puddles was best. We now know that it is richest in the minerals necessary for plant growth, whereas rainwater is almost completely lacking in them.
A problem that impeded study of plants and animals was the belief that living creatures contain a vital force, something that was not chemical or physical and was assumed to be beyond study. It was believed that when an organism died and decayed, its vital force passed into the soil and made it fertile. Try to think like someone in 1700, only 50 years after the Pilgrims landed at Plymouth Rock. You would know that soil could be fertilized with bonemeal, manure, fish scraps, or compost (TABLE B13-3). All of these things had been living and could presumably add vital force to the soil.
At this time, two scientists, Nehemiah Grew and Marcello Malpighi, were making the first studies of the microscopic structure of plants and animals. Unfortunately, some of the early observations were misinterpreted and reinforced the concept of the existence of vital force. For example, the presence of microscopic holes in various types of cells caused people to think in terms of filtration: It was postulated that roots contained fine pores that allowed water and vital humours (liquids) to pass into the plant while non-nutritive soil sap was excluded. After partially purified by root filtration, the vital humours from the soil were thought to be transported upward, being further filtered by the pits and perforations of the xylem cells. This filtration presumably purified the vital force, allowing it to be transmuted into plant cells. This incorrect hypothesis misled scientists, and little thought was given to the role of minerals, which seemed too simple to be very important for life.
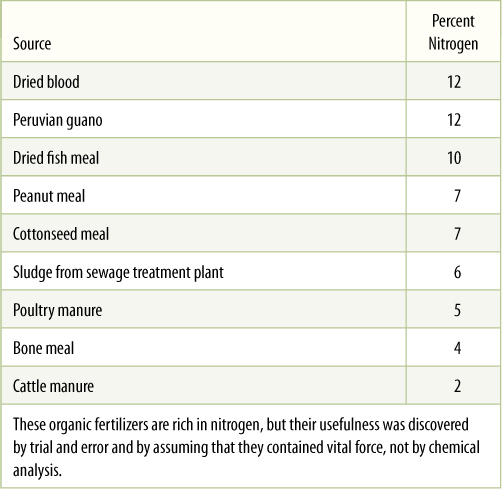
TABLE B13-3 Natural Sources of Organic Nitrogen
Modern analysis of plant and animal mineral nutrition was further hindered by another incorrect concept—that of spontaneous generation. It was believed that the vital force of dead plants and animals, if not absorbed by plant roots, would transform itself directly into new plants and animals. When vital force became sufficiently concentrated in soil, it was thought to cause worms, ants, mushrooms, and ferns to come into existence spontaneously. For example, the vital force of a dead animal was believed to cause the spontaneous generation of maggots and that of dead trees to produce mushrooms. Microscope studies would not reveal that maggots develop from fly eggs and mushrooms are composed of fungal filaments until years later.
In the 1800s, several important discoveries laid the foundation of modern biological studies based on chemical and physical principles. The first breakthrough came with the work of N. T. de Saussure in 1804; by this time, it was possible to prepare or purchase many chemical compounds in rather pure and well-defined condition. De Saussure designed and carried out the first well-planned hydroponics experiment and proved that nitrogen is essential for plant growth and development. The work of Julius Sachs followed, establishing the basic concepts of mineral nutrition in plants. It is difficult for us to appreciate how important these botanical discoveries were, but imagine the impact on 19th century scientists: Plants could be grown to maturity in the laboratory using no soil or other natural products. Containers, solutions, even the newly discovered electric lights, were all man made and humanly controlled. Hydroponic experiments established that life, as exemplified by plant life, is described by the laws of physics and chemistry, not metaphysics. Furthermore, contemporaneous with Sach’s research, the theory had just been formulated that cells come only from preexisting cells, and in the late 1800s, Louis Pasteur proved that no vital force exists and that spontaneous generation does not occur. At approximately the same time, the first artificial synthesis of a biological compound, urea, proved that vital force was unnecessary in the construction of the material of protoplasm. The 1800s were exciting times for the philosophy of science and the concept of our relationship with nature. The study of metabolism became a science based completely on chemistry, physics, and mathematics; metaphysics and mysticism were eliminated.
Apparently, all parts of a plant except for seeds store minerals in soluble form in the central vacuoles of cells. Nitrogen can be concentrated a little by being converted to compounds with multiple amino groups such as asparagine, citrulline, and so on, the same compounds that are used in nitrogen transport. Phosphates, sulfates, and other mineral nutrients apparently are simply sequestered in the central vacuole in the same forms in which they are used metabolically by the cell. This method does not permit storage of large amounts of minerals because high concentrations would be toxic, but plant cells differ from our cells by having very large vacuoles and just a tiny amount of cytoplasm squeezed between the vacuole membrane and the cell membrane. Pound for pound, plant tissues need only very small amounts of minerals compared with animals.
In contrast, seeds do store minerals. Seeds must be both lightweight but also packed with enough resources to get a seedling established quickly. Amino acids are stored as particles of protein, protein packed so tightly that it typically crystallizes into a structure called a protein body. In many seeds, protein bodies themselves contain inclusions of another crystalline form, crystals of a substance called phytin (technically, myo-inositolhexaphosphate). Myo-inositol-hexaphosphate is a six-carbon sugar that has six hydroxyl groups, each carrying a phosphate bound to it. Being an acid, myoinositol-hexaphosphate ionizes and loses protons, H+, when dissolved in water, but when the developing seed concentrates it and precipitates it in the protein body, it puts cations on it rather than returning the protons, and the cations used are Mg2+, Ca2+, Zn2+, and K+. This mineral-holding form is phytin, and it permits the protein body to not only store amino acids and phosphate but all of these other essential elements as well.

FIGURE 13-19 (A) A cultivated ant-plant, Hydnophytum, with a greatly enlarged tuberous hypocotyl. Holes visible on the surface form naturally and give ants access to chambers inside the hypocotyl. (B) A young plant of Hydnophytum, cut open longitudinally to show the chambers that develop naturally inside. Because this is cultivated in a greenhouse that controls insects and other small animals, no ants live in these chambers. (C) The enlarged hypocotyl of another ant-plant, Myrmecodia; many small entrance holes are visible. (D) This fern, Lecanopteris carnosa, is also an ant-plant, having swollen, hollow chambers in its stem.
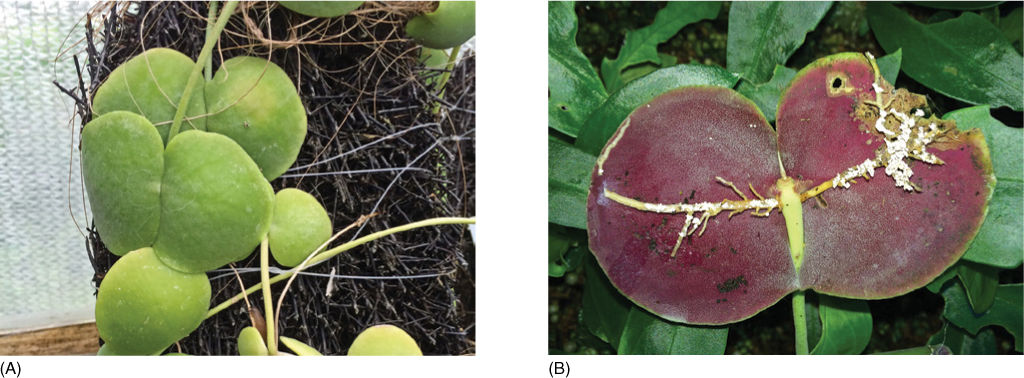
FIGURE 13-20 (A) Plants of Dischidia major are also ant-plants. They form two types of leaves: Some are cup-like and clasp a tree trunk or branch; others are hollow, balloon-shaped leaves. Ants live in both types and debris accumulates. The Dischidia stem sends adventitious roots (B) into the debris trapped by the leaves, absorbing nutrients from it. This plant is cultivated in a greenhouse too clean to have ants.
Plants and People
BOX 13-4 Fertilizers, Pollution, and Limiting Factors
Plants in nature usually do not grow as vigorously as they potentially could. For example, desert plants typically grow more rapidly if given extra water, plants in shady areas grow better if given a bit more light, and prairie grasses, which already have enough water and light, benefit from extra nitrogen fertilizer. Any plant grows at a particular speed and vigor because it is limited by some factor, such as too little water or light or nitrogen fertilizer. An important concept is that there is only one single limiting factor at a time for any plant. Desert plants given more light or fertilizer will not grow faster—it is water that is limiting them. But if we do give them extra water, their growth rate will increase until some other factor becomes limiting, perhaps lack of nitrogen. While growing slowly, the plants could get nitrogen quickly enough, but now that they are growing faster, their ability to obtain nitrogen from the soil may be limiting. If we give the plants both water and nitrogen fertilizer, they may grow even more rapidly, but some other factor will become limiting. On many farms plants are irrigated and fertilized and planted far enough apart that they do not shade each other and their roots do not interfere with each other. Such plants grow much more rapidly than they would in nature, but they are still limited, in this case, by their own genetics, their own innate metabolic capacity. There is always a limiting factor.
The concept of a limiting factor is important in understanding techniques for reducing the damage caused by pollution. Under natural conditions the water in rivers and lakes has so few nutrients that algae grow slowly, and they are so sparse the water is blue. In the mid-20th century pollution from farms and cities fertilized rivers and lakes and allowed algae to grow more vigorously. As rain drained from fields, lawns, gardens, and golf courses, it carried much of the fertilizers that had been applied to stimulate the growth of crops, flowers, and grass. Also, most household waste that is flushed down toilets is an excellent organic fertilizer. With all these extra inputs of nutrients, populations of algae became so dense that “pond scum” floated near the surface of rivers and lakes, and the water was green because of the abundance of microscopic algae (FIGURE B13-4A).
It is difficult to stop all pollution, but it is only necessary to control one single pollutant to create a limiting factor. Phosphate was chosen as the target. Phosphorus is an essential element, and it is naturally low in pure water. A large amount of the phosphate pollution in rivers comes from laundry detergent and dishwashing soap. With a little effort phosphate-free detergents were invented, and now they are used almost universally, so there is much less phosphate pollution. The concentration of phosphate in rivers dropped so low that algae could no longer thrive, and their populations fell to more normal levels. Even though the water is still heavily polluted with nitrates, sulfates, and other nutrients, the algae cannot use them as long as phosphate is kept low enough to limit their growth. If we could reduce the phosphate runoff from farms and lawns, the levels of algae would drop even more and rivers and lakes would be even cleaner (FIGURE B13-4B). It would be better to control and reduce all types of pollutants, but by keeping one at limiting levels we can at least minimize some of the damage caused by pollution.

FIGURE B13-4 (A) This river runs through extensive agricultural areas in east Texas and is polluted by fertilizers from fields and manure from livestock; because the water is so rich in nutrients, algae and bacteria grow well, giving the water a brown color. (B) This stream in North Carolina runs through a forested area without agriculture, so it does not receive as much pollution as the river in (A). There are many towns and homes along this river, but because modern detergents do not contain phosphates, the wastewater dumped into this river is almost phosphate-free, so algae and other microbes cannot grow in it and the river has clear, blue water.
 At the Next Level
At the Next Level
1. Composting. Many people are trying to live more harmoniously with the other creatures on Earth and are trying to reduce the harm we do. One aspect of this is to use natural, organic fertilizers rather than artificial, inorganic commercial fertilizers. Most organic material must be composted before it can be used: In composting, the materials are collected into a large pile (at least 5 or 10 m3) and allowed to “ferment” for weeks or months. Enzymes and microbes break down complex molecules, freeing the minerals. The fermentation causes the compost pile to become hot enough to kill harmful bacteria, which is important if the compost contains manure or animal parts. The process of composting is easy to do at home and it involves complex biology so it is an excellent topic for independent study at the next level.
2. Mineral nutrition and climate change. Increased greenhouse gases cause the oceans as well as the land to become warmer. This causes more oceanic evaporation, which later falls as rain. Every time rain water runs off into streams and rivers, it carries some minerals with it, washing them out of the soil. If the area has a large amount of dead plant material, for example as leaf litter and humus, this helps to retain the minerals, but desert areas have little organic matter in the soil, and when they receive more rain, they lose more minerals. As Earth warms and rains increase, soil leaching will also increase, affecting the fertility of the soil and ecology of regions. A key word is biogeochemistry.
3. Hydroponics. Hydroponics is the technique of growing plants without soil. Originally, plants were grown with their roots in aqueous solutions of dilute fertilizer, but now some techniques involve merely misting roots periodically to keep them moist and nourished. By growing plants hydroponically, we can control their water and fertilizer precisely, and eliminate soil-associated pests such as nematodes. For space exploration, using hydroponic misting allows plants to be cultivated without the weight of either tanks of water or soil. Experiments are being performed on the International Space Station. Hydroponics will be especially important when we send manned flights to Mars and must not only grow some fresh food but also recycle water and re-use human urine and feces by means of composting. Some of these problems are discussed in the book Packing for Mars: The Curious Science of Life in the Void by Mary Roach (2010, WW Norton). I strongly recommend this book as an enjoyable, humorous, and informative introduction into the problems of biology in microgravity.
SUMMARY
1. Plants synthesize all their body’s materials using only carbohydrate and a few essential elements.
2. Three criteria must be met for an element to be considered essential: (1) It is necessary for the completion of a full life cycle; (2) it cannot be replaced by a chemically similar element; and (3) it must act inside the plant.
3. Macro or major essential elements are needed in relatively large amounts, whereas micro or minor or trace elements are required in only extremely small amounts.
4. Deficiency diseases are rare in nature, probably because susceptible plants are outcompeted by deficiency-tolerant plants; however, deficiency symptoms are frequent in crops and horticultural plants if proper fertilizing is not maintained.
5. Deficiency of an immobile element produces symptoms in young leaves and buds, whereas deficiency of a mobile element results in symptoms in older organs.
6. Cations are released from the surface of soil particles if protons, which result from the respiration of roots and soil organisms, disrupt their electrical attraction.
7. Mycorrhizae are the principal means by which most plants absorb phosphorus.
8. Nitrogen metabolism consists of fixation, reduction, and assimilation.
9. Nitrogen must be obtained from the air, but only certain prokaryotes—some free living, others symbiotic—have the necessary enzyme, nitrogenase.
10. Most plants absorb nitrate, which must be reduced by the plants, using large quantities of NADH, NADPH, and ferredoxin.
11. Once formed within plant cells, ammonium is assimilated by transamination, being passed from glutamate to various amino acids.
IMPORTANT TERMS
acid rain
amino group
carnivorous plants
cation exchange
chlorosis
denitrifying bacteria
domatium
essential elements
eutrophication
field capacity
hydroponic solution
immobile elements
major (macro) essential elements
micelles
mineral nutrition
minor (micro) essential elements
mobile elements
necrosis
nitrifying bacteria
nitrogen assimilation
nitrogen fixation
nitrogen reduction
trace elements
transamination
weathering
REVIEW QUESTIONS
1. Most elements that are essential for plant growth are present in the____________ ____________ ____________ ____________. How do the elements become available?
2. What is meant by the microflora and microfauna of the soil? Why are they important?
3. How many bacteria typically occur in one gram of soil?
4. What is a hydroponic solution? After a solution of known composition which supports plant growth is found, how would you go about testing whether all of the chemicals in the solution are necessary for plant growth?
5. It is simple to grind up a plant and then extract and measure all of the chemical elements present, but this does not tell us which elements are essential. Why not?
6. List the major or macro essential elements. Why are they called that?
7. List the minor or micro (trace) essential elements. Why are they called that?
8. Are our modern reagents so pure that we can prepare a hydroponic solution that contains only the nine major and seven minor essential elements and nothing else? Can we be certain we have discovered all the minor essential elements?
9. What are the three criteria an element must meet to be considered essential?
10. Do you think anyone has performed a mineral nutrition experiment that examined the entire life cycle of a giant redwood? A giant cactus? A mistletoe that is parasitizing an oak tree? What would be the problems involved?
11. To be essential, an element must be acting within the plant, not outside it. What does this mean? Discuss the roles of iron and phosphorus in determining which elements are essential for a plant.
12. We humans, like all other organisms, use a universal set of twenty amino acids in our proteins, but we cannot make ______________ of these ourselves. How many are plants unable to make? How many do plants need to take up through their roots?
13. The differences in nutritional resources used by plants versus animals is also great. Plants obtain nutrients in the form of __________________ or as simple ______________ present in the environment. An animal begins with food in the mouth, but the nutrients occur as _________________ in complex _________________ which in turn are parts of ________________.
14. Name the essential element involved in each process: changes the osmotic potential in guard cells as they swell or shrink, present in all nucleotides and amino acids, carries electrons in chlorophyll, present in cytochromes, present in plastocyanins, involved in nitrogen metabolism, present in the water-splitting enzyme of photosystem II, present in ATP, present in the middle lamella, and important in regulating the activity of many enzymes.
15. Under natural conditions, is it rare or common to encounter plants whose growth is disrupted by a scarcity or an excess of mineral elements? Describe two habitats that often have excessive amounts of mineral elements (Hint: do not forget to study the figures as well as the text).
16. Question 15 specified natural conditions. What about nonnative crop plants and ornamentals? Do they often encounter mineral deficiencies?
17. Name and describe two common symptoms of deficiency diseases.
18. What is the difference between a mobile and an immobile element? Where are the first deficiency symptoms for each? Which elements are immobile?
19. Do we understand why certain minerals are immobile in plants? What is one essential element that we humans do not recycle well in our own bodies?
20. What are the two fundamental processes of weathering that convert rock to soil?
21. What are size ranges of particles of coarse sand, fine sand, silt, and clay particles (also called clay micelles)? Coarse sand can absorb a great deal of water during a rain, but what happens to the water soon after the rain stops? What would happen instead if the soil consisted of clay micelles?
22. Chemical weathering dissolves the surface of the crystal matrix of rock. As the matrix breaks down, what is released—positively charged cations or negatively charged anions? As a result, the residual crystal matrix has what kind of charge?
23. Many factors affect soil acidity, but probably the most important factor of all is _______________. Describe how this factor exerts its effect.
24. Most soils are ______________ to ______________ acidic. Desert soils, which have very little leaf litter or other organic matter, are usually _______________ (_____________________).
25. Describe cation exchange. How would decomposition of mulch and humus affect cation exchange? Why is acid rain so damaging?
26. The roots of most plants form a symbiotic association with soil fungi. Name the association and the element that it supplies to the plant.
27. Which essential element is neither a component of rock matrixes nor a contaminant in rock? Although this element is abundant in air, no plant or animal can absorb it from the air and use it. Why not?
28. Name the three steps in the conversion of N2 into organic nitrogen that is part of a plant. Which type of organism is capable of performing each step?
29. What is nitrogenase? What chemical does it use as a substrate? It is extremely sensitive to ________________ and can function only if _______________ is completely excluded from it.
30. The electrons that reduce nitrate to nitrite are brought to it by a short electron transport chain involving FADH2 and NADH (see Figure 13-16). In which other reactions have you seen these two electron carriers?
31. How does nitrogen reduction affect carbon fixation in the stroma reactions? How many molecules of NADPH must be diverted from one pathway to the other? How would respiration be affected?
32. What is nitrogen assimilation? What is the acceptor molecule? What does it react with? What is the product?
33. What is transamination? If an amino group is passed onto oxaloacetate, which amino acid is produced? If an amino group is transaminated onto pyruvate, which amino acid is produced?
34. Figure 13-18 shows several chemicals that carry nitrogen up from the roots to the rest of the plant. What is the unusual feature of these chemicals that make them good carriers?
Design Credits: Hummingbird: © Tongho58/Moment/Getty; Green plant cells: © ShutterStock, Inc./Nataliya Hora; Purple tulip: © ShutterStock, Inc./Marie C Fields; Dandelion: © ShutterStock, Inc./danielkreissl; Poppy: © ShutterStock, Inc./Saruri; Plant icon: © ShutterStock, Inc./Vector; Digging man icon: © ShutterStock, Inc./Z-art