CONCEPTS IN BIOLOGY
PART V. THE ORIGIN AND CLASSIFICATION OF LIFE
19. The Origin of Life and the Evolution of Cells
19.4. The Chemical Evolution of Life on Earth
When we consider the nature of the simplest forms of life today, we find that living things have certain characteristics. They have an outer membrane, which separates the cell from its surroundings; genetic material in the form of nucleic acids; and many kinds of enzymes, which control the activities of the cell. Therefore, when we speculate about the origin of life from inorganic material, it seems logical that several events or steps were necessary:
1. Simple organic molecules must first have been formed from inorganic molecules.
2. Simple organic molecules must have combined to form larger organic molecules, such as RNA, proteins, carbohydrates, and lipids.
3. A molecule must have served as genetic material.
4. Genetic material must have become self-replicating.
5. Some molecules must have functioned as enzymes.
6. The molecules serving as genetic material and other large organic molecules must have been collected and segregated from their surroundings by a membrane.
7. The first life-forms would have needed a way to obtain energy from their surroundings in order to maintain their complex structure.
The Formation of the First Organic Molecules
In the 1920s, a Russian biochemist, Alexander I. Oparin, and a British biologist, J. B. S. Haldane, working independently, proposed that the first organic molecules were formed spontaneously in the reducing atmosphere thought to be present on the early Earth. According to their theory, inorganic molecules in the primitive atmosphere supplied the atoms of carbon, hydrogen, oxygen, and nitrogen needed to build organic molecules. Lightning, heat from volcanoes, and ultraviolet radiation furnished the energy needed for the synthesis of simple organic molecules from inorganic molecules (figure 19.5).
It is important to understand the significance of a reducing atmosphere to this proposed mechanism for the origin of life. The absence of oxygen in the atmosphere would have allowed these organic molecules to remain and combine with one another. This does not happen today because organic molecules are either consumed by organisms or oxidized to simpler inorganic compounds because of the oxygen present in our atmosphere. For example, today many kinds of organic air pollutants (hydrocarbons) eventually oxidize into smaller molecules in the atmosphere. Unfortunately, they participate in the formation of photochemical smog as they are broken down.
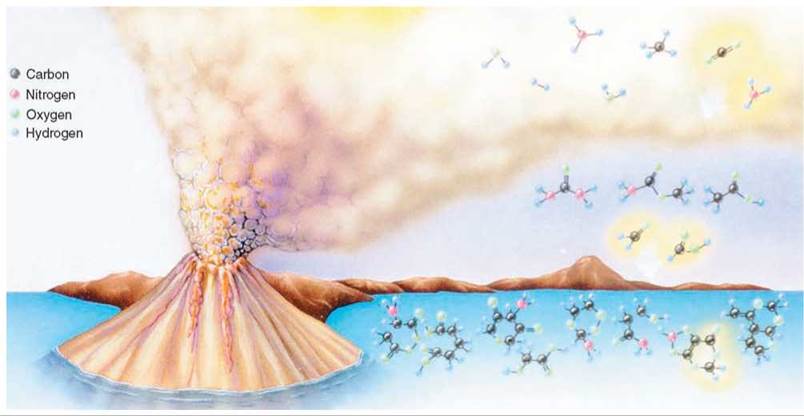
FIGURE 19.5. The Formation of Organic Molecules in the Atmosphere
The environment of the primitive Earth was harsh and lifeless. But many scientists believe that it contained the necessary molecules and sources of energy to fashion the first living cell. The energy furnished by volcanoes, lighting, and ultraviolet light could have broken the bonds in the simple inorganic molecules in the atmosphere. New bonds could have formed as the atoms from the smaller molecules were rearranged and bonded to form simple organic compounds in the atmosphere. Rain and runoff from the land would have carried these chemicals into the oceans, where they could have reacted with each other to form more complex organic molecules.
Recognize that all the ideas discussed so far in this section cannot be confirmed by direct observation, because we cannot go back in time. However, several assumptions central to this model for the origin of life have been laboratory tested.
In 1953, Stanley L. Miller conducted an experiment to test the idea that organic molecules can be synthesized in a reducing environment. He constructed a simple model of the early Earth’s atmosphere (figure 19.6). In a glass apparatus, he placed distilled water to represent the early oceans. Adding hydrogen (H2), methane (CH4), and ammonia (NH3) to the water (H2O) simulated the reducing atmosphere. Electrical sparks provided the energy needed to produce organic compounds. By heating parts of the apparatus and cooling others, he simulated the rains that are thought to have fallen into the early oceans. After a week of operation, he removed some of the water from the apparatus. When he analyzed this water, he found that it contained many simple organic compounds.
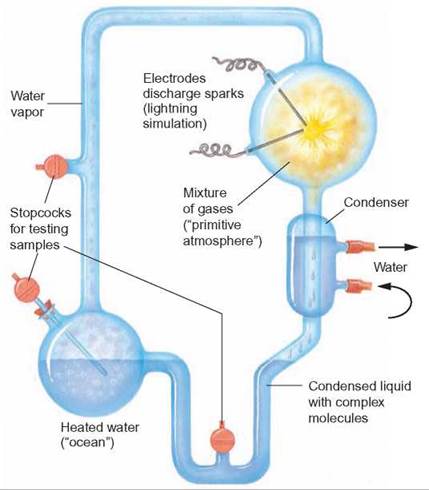
FIGURE 19.6. Miller's Apparatus
Stanley Miller developed this apparatus to demonstrate that the spontaneous formation of organic molecules from inorganic molecules could take place in a reducing atmosphere.
The idea that organic molecules could have formed on Earth is further supported by the discovery of many kinds of organic molecules in interstellar clouds and in the structure of meteorites. These pieces of evidence show that organic molecules form without the presence of living things.
The Formation of Macromolecules
Although Miller demonstrated the nonbiological synthesis of simple organic molecules, such as amino acids and simple sugars, his results did not account for complex organic molecules, such as proteins and nucleic acids (e.g., DNA).
After simple organic molecules were formed in the atmosphere, they probably would have been washed from the air and carried into the newly formed oceans by rain and runoff from the land. There, the molecules could have reacted with one another to form the more complex molecules of simple sugars, amino acids, and nucleic acids. The accumulation of larger organic molecules is thought to have occurred over half a billion years, resulting in oceans that were a dilute organic soup.
Several ideas have been proposed to explain how simple organic molecules could have been concentrated and caused to combine to form larger macromolecules. One hypothesis suggests that a portion of the early ocean could have been separated from the main ocean by geologic changes. The evaporation of water from this pool could have concentrated the molecules, which might have led to the formation of macromolecules by dehydration synthesis. Second, it has been proposed that freezing may have been the means of concentration. When a mixture of alcohol and water is placed in a freezer, the water freezes solid and the alcohol becomes concentrated into a small portion of liquid. A similar process could have occurred on Earth’s early surface, resulting in the concentration of simple organic molecules. In this concentrated solution, dehydration synthesis in a reducing atmosphere could have occurred, resulting in the formation of macromolecules. A third theory proposes that clay particles may have been a factor in concentrating simple organic molecules. Small particles of clay have electrical charges, which can attract and concentrate organic molecules, such as proteins, from a watery solution. Once the molecules became concentrated, it would have been easier for them to interact to form larger macromolecules.
RNA May Have Been the First Genetic Material
As you know from chapter 8, the genetic system of most current organisms involves the replication of DNA and the distribution of the copied DNA to subsequent cells. Furthermore, DNA is responsible for the manufacture of RNA, which is responsible for the manufacture of proteins. Some of the proteins produced serve as enzymes that control chemical reactions. However, it is difficult to see how this complicated sequence of events, which involves many steps and the assistance of several enzymes, could have been generated spontaneously, so scientists have looked for simpler systems that could have led to the current DNA system.
In recent years many people have come to look at RNA as the prime candidate for the first genetic material. In order to serve as genetic material, a molecule must store information, mutate, and make copies of itself:
1. RNA can store genetic information. Scientists involved in studying the structure and function of viruses discovered that many viruses do not contain DNA but, rather, store their genetic information in the structure of RNA. In order for these RNA-viruses to reproduce, they must enter a cell. The cell makes copies of the RNA and the proteins necessary to make more of the virus. Certain plant diseases are caused by pieces of naked RNA known as viroids, which enter cells and cause the cells to make copies of the RNA. In other words, in both of these cases, RNA serves as genetic material.
2. RNA can mutate. Scientists who study viral diseases find that it is difficult to develop vaccines for many viral diseases because their genetic material mutates easily. Because of this, researchers have been studying the nature of viral DNA or RNA to see what causes the high rate of mutation.
3. RNA can make copies of itself. RNA can be assembled from simpler subunits that could have been present on the early Earth. Scientists have also shown that RNA molecules are able to make copies of themselves without the need for enzymes, and they can do so without being inside cells. Furthermore, some RNA molecules serve as enzymes for specific reactions. Such molecules are called ribozymes. Because RNA is a much simpler molecule than DNA, contains genetic information, can mutate, and can make copies of itself without the aid of enzymes, perhaps it was the first genetic material. Once a primitive life-form had the ability to copy its genetic material, it would be able to reproduce. Reproduction is one of the most fundamental characteristics of living things.
If RNA was the first genetic material, many subsequent changes would have been necessary to get to the kind of genetic system we see in most organisms today:
1. DNA must have replaced RNA as the self-replicating genetic material of the cell.
2. DNA must have become the molecule responsible for making RNA.
3. RNA must have taken over control of protein synthesis.
4. Proteins must have become the catalysts (enzymes) of the cells.
5. Membranes and other cellular structures involved in cell reproduction and protein synthesis would have developed.
Obviously, there is much still to learn about the how this genetic system developed.
The Development of Membranes
One of the defining features of any living thing is the presence of a membrane surrounding its cells which regulates what enters and leaves them. Consider the formation of bubbles in water. Bubbles are particularly common when organic molecules are present in water. Perhaps the first membranes were formed because of an interaction between water and organic molecules similar to the formation of bubbles. There are several theories about what the first membranes were like. Some suggest that they were made of proteins, others that they were lipids or other organic molecules. Several kinds of experiments have sought to clarify what the first membrane might have been like. Alexander I. Oparin mentioned earlier, speculated that a structure could have formed consisting of a collection of organic macromolecules surrounded by a film of water molecules. This arrangement of water molecules, although not a membrane, could have functioned as a physical barrier between the organic molecules and their surroundings. He called these structures coacervates.
Coacervates have been synthesized in the laboratory (figure 19.7). They can selectively absorb molecules from the surrounding water and incorporate them into their structure. Also, the chemicals within coacervates have a specific arrangement— they are not random collections of molecules. Some coacervates contain proteins (enzymes) that direct a specific type of chemical reaction. Because they lack a definite membrane, no one claims that coacervates are alive, but these structures do exhibit some lifelike traits: They can increase in size and can split into smaller particles if the right conditions exist.
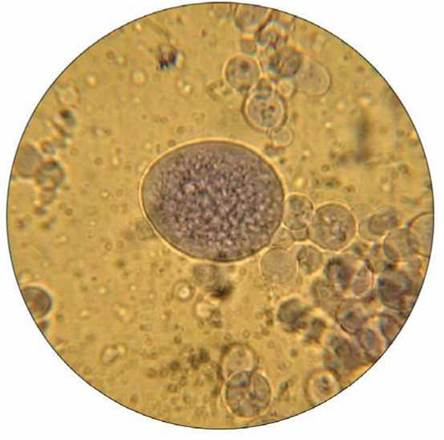
FIGURE 19.7. Coacervates
One hypothesis proposes that a film of water, which acted as a primitive cell membrane, could have surrounded organic molecules, forming a structure that resembles a living cell. Such a structure can easily be produced in the lab. This view shows one large and several small spherical coacervates.
An alternative hypothesis is that the early, cell-like structure could have consisted of a collection of organic macromolecules with a double-layered outer boundary. These structures have been called microspheres. Microspheres can be formed in the laboratory by heating simple, proteinlike compounds in boiling water and slowly cooling them. Some of the protein material produces a double-boundary structure enclosing the microsphere. Although these boundaries do not contain lipids, they exhibit some membranelike characteristics and suggest the structure of a cellular membrane.
Microspheres swell or shrink, depending on the osmotic potential in the surrounding solution. They also display a type of internal movement (streaming) similar to that exhibited by living cells and contain some molecules that function as enzymes. Using ATP as a source of energy, microspheres can direct the formation of polypeptides and nucleic acids. They can absorb material from the surrounding medium and form buds, resulting in a second generation of microspheres.
A third possibility is that a membrane forms as a result of lipid materials interacting with water. Lipids do not dissolve in water and whenever lipids are mixed with water, spherical structures form, as in vinegar and oil salad dressing.
There is no way to know if any of these models represents what really happened in the origin of living things. However, some kind of structure was necessary to separate the complex, organized molecules from the watery environment in which they were dissolved. Once organic molecules were separated from their watery surroundings by a membrane, these structures were similar to primitive cells.
The Development of Metabolic Pathways
Fossil evidence indicates that there were primitive forms of life on Earth about 3.5 billion years ago. Regardless of how they developed, these first primitive cells would have needed a way to add new organic molecules to their structures as previously existing molecules were lost or destroyed. There are two ways to accomplish this. Heterotrophs capture organic molecules, such as sugars, amino acids, or organic acids, from their surroundings, which they use to make new molecules and provide themselves with energy. Autotrophs use an external energy source, such as sunlight or the energy from inorganic chemical reactions, to combine simple inorganic molecules, such as water and carbon dioxide, to make new organic molecules. These new organic molecules can then be used as building materials for new cells or can be broken down at a later date to provide energy.
The Heterotroph Hypothesis
Many scientists support the idea that the first living things on Earth were heterotrophs, which lived off organic molecules in the oceans. There is evidence to suggest that a wide variety of compounds were present in the early oceans, some of which could have been used, unchanged, by primitive cells. The earliest cells appear in the fossil record over 2 billion years before there is evidence of oxygen in the atmosphere. Therefore, these early heterotrophs would have been anaerobic organisms. However, as their populations increased through reproduction, they would have begun to consume organic molecules faster than they were being spontaneously produced in the atmosphere.
The compounds that could be used easily by these cells would have been the first to become depleted. However, some of the heterotrophs may have contained a mutated form of nucleic acid, which allowed them to convert previously unusable material into something they could use. Heterotrophic cells with such mutations could have survived, whereas those without such mutations would have become extinct as the compounds they used for food became scarce. It has been suggested that, through a series of mutations in the early heterotrophic cells, a more complex series of biochemical reactions originated within some of the cells. Such cells could use chemical reactions to convert unusable chemicals into usable organic compounds. Thus, additional steps would have been added to their metabolic processes, and new metabolic pathways would have evolved.
The Autotroph Hypothesis
The heterotroph hypothesis for the origin of living things was the prevailing theory for many years. However, recent discoveries have caused many scientists to consider an alternative—that the first organisms were autotrophs. Several kinds of information support this theory. There is much evidence that Earth was a much hotter place in the past. Today, many different kinds of prokaryotic organisms are autotrophic and live in extremely hostile environments resembling the conditions that may have existed on the early Earth. These organisms are found in hot springs—such as those found in Yellowstone National Park; in Kamchatka, Russia (Siberia); and near thermal vents—areas where hot, mineral- rich water enters seawater from the deep ocean floor. They use energy released from inorganic chemical reactions to synthesize organic molecules from inorganic components. Because of this, they are called chemoautotrophs. If their ancient ancestors had similar characteristics, the first organisms may have been autotrophs.
If the first organisms were autotrophs, there would have been competition among different cells for the inorganic raw materials they needed for their metabolism, and there would have been changes in the metabolic processes as mutations occurred. There could have been subsequent evolution of a variety of cells, both autotrophic and heterotrophic, which could have led to the diversity of prokaryotic organisms seen today.
Summary
As a result of this discussion you should understand that we do not know how life on Earth originated. Scientists look at many kinds of evidence and continue to explore new avenues of research. Thus, currently there are three competing theories of the origin of life on Earth:
1. Life arrived here from an extraterrestrial source.
2. Life originated on Earth as a heterotroph.
3. Life originated on Earth as an autotroph.
Figure 19.8 summarizes the steps that are thought to have been necessary for primitive cells to evolve from inorganic molecules.
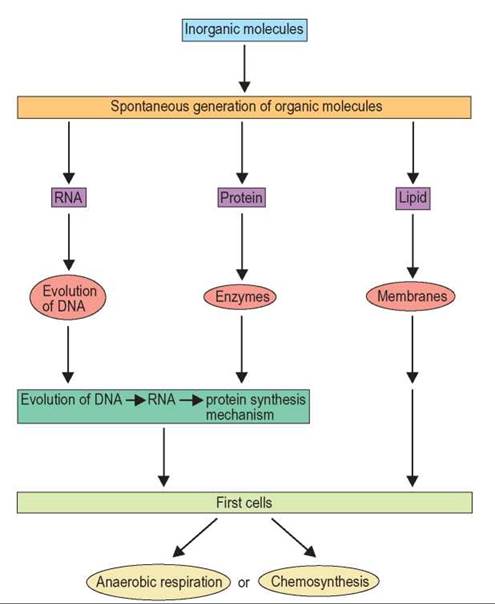
FIGURE 19.8. The Chemical Evolution of Life
This diagram summarizes the steps necessary for primitive cells to evolve from inorganic molecules.
19.4. CONCEPT REVIEW
8. List two kinds of evidence that suggest that organic molecules could have formed before there were living things.
9. List two kinds of evidence that suggest that RNA was the first genetic material.
10. Describe two models that suggest how collections of organic molecules could have been segregated from other molecules.
11. Why must the first organism of Earth have been anaerobic?
12. How do heterotrophs and chemoautotrophs differ in how they obtain organic molecules?