CONCEPTS IN BIOLOGY
PART VI. PHYSIOLOGICAL PROCESSES
25. Nutrition. Food and Diet
25.2. The Kinds of Nutrients and Their Function
Nutritionists have divided nutrients into six major classes: carbohydrates, lipids, proteins, vitamins, minerals, and water. Chapters 2 and 3 presented the chemical makeup of these types of molecules, and chapter 6 explored the nature of cellular respiration. A look at each of these classes of nutrients from a nutritionist’s point of view should reveal how the human body works and how its nutritional needs can be met.
Carbohydrates
From a nutritional point of view, there are three kinds of carbohydrates that are significant: sugars, starch, and fiber. The basic building blocks of carbohydrates are simple sugars (monosaccharides) such as glucose, fructose, and galactose. Two simple sugars can be combined to form double sugars (disaccharides) such as lactose, maltose, and sucrose. Both mono- and disaccharides are commonly called sugar. In plants, large numbers of glucose molecules can be joined to form a polysaccharide called starch. The function of starch in the plant is to store food. However, animals can use this energy-storage molecule for energy as well. Starch is a primary source of Calories for humans (figure 25.2).

FIGURE 25.2. Carbohydrates
The primary source of carbohydrates is seeds of plants such as wheat, oats, rice, and beans and things made from the seeds. For most of the people of the world, carbohydrate in the form of starch is the primary item in their diet.
Functions of Carbohydrates
The primary function of sugars and starch in the diet is as a source of energy. During digestion, starch and disaccharides are broken down to simple sugars that are absorbed from the gut and used by cells during respiration. There are 4 Calories (kilocalories) in a gram of carbohydrate. In addition to serving as a source of energy, most sugars (glucose, fructose, lactose, sucrose, maltose) taste sweet and stimulate the appetite. Simple sugars are also used as building blocks in the manufacture of molecules such as nucleic acids.
Dietary fiber consists of cellulose and several other complex carbohydrates that are indigestible. Dietary fiber has several nutritional functions. It slows the absorption of sugars, which helps to regulate the level of glucose in the blood. This is particularly important for diabetics. Dietary fiber also reduces the absorption of cholesterol from the intestine and thus can have the beneficial effect of lowering cholesterol in the blood, which reduces the incidence of heart disease. It provides bulk to the contents of the intestine and stimulates peristalsis (rhythmic contractions) in the intestinal tract. It also tends to retain water in the intestine, reducing the incidence of constipation.
Carbohydrate Quality
Food sources that contain carbohydrates are not all the same. Flour is made from seeds such as wheat, corn, rye, rice, buckwheat, and millet that are ground up. Many kinds of foods are constructed from refined flour, which is essentially pure starch. Whole-grain flour contains many other parts of the seeds and has much more fiber and other nutrients than contained in refined flours. Foods that consist primarily of refined flours and sugars (candy bars, soft drinks, white bread, cakes, etc.) are high in Calories but low in other important nutrients. Therefore, these foods sources are often said to have “empty Calories”—they provide Calories but little other nutritional value. The latest nutritional recommendations encourage people to reduce their consumption of refined flours and sugars and increase their consumption of whole grain products.
How the Body Manages Carbohydrates
When we consume carbohydrates, we cannot use them immediately to provide energy for our cells. Our bodies store this energy in two ways. We can store small amounts as glycogen in muscles and the liver for later use. Glycogen consists of glucose molecules hooked together but in a different way from that of starch. Glycogen is sometimes called animal starch. The body can only store small amounts of carbohydrate as glycogen. Therefore, carbohydrates should be a daily part of the diet. If we consume much more carbohydrate than we need, the excess is converted to fat and stored in fat tissue.
A diet deficient in carbohydrates results in fats being oxidized and converted to ATP. In situations in which carbohydrates are absent, most of the fats are metabolized to keto acids. Large numbers of keto acids may be produced in extreme cases of fasting, resulting in a potentially dangerous change in the body’s pH. If a person does not have stored fat to metabolize, a carbohydrate deficiency will result in use of the body’s proteins as a source of energy. This is usually only encountered in starvation, extreme cases of fasting, or eating disorders. In extreme cases, this can be fatal, because the oxidation of protein results in an increase in toxic, nitrogen-containing compounds.
Lipids
The class of nutrients technically known as lipids is often called fats. This may lead to some confusion, because fats are only one of three subclasses of lipids. Each subclass of lipids— phospholipids, steroids, and true fats—plays an important role in human nutrition.
Phospholipids are the major molecules in membrane structures (endoplasmic reticulum, plasma membrane, Golgi, etc.) of cells. Although various kinds of phospholipids are sold as dietary supplements, they are unnecessary because all food composed of cells contains phospholipids.
Many steroids are hormones that help regulate a variety of body processes. With the exception of vitamin D, it is not necessary to include steroids as a part of the diet. Cholesterol is a steroid, manufactured in the body and commonly found in foods of animal origin. Excess consumption of cholesterol causes health problems in some people.
The true fats (also called triglycerides) are composed of a glycerol molecule attached to three fatty acids.
Functions of Fats
Fats are the primary long-term, energy-storage molecules in animals and plants. Therefore, they are an excellent source of energy. They release 9 Calories of energy per gram compared to 4 Calories per gram for carbohydrate or protein. A teaspoon holds about 5 grams of a fat like olive oil.
In addition to serving as sources of energy in the diet and energy-storage molecules in the body, fats have several other important functions. Some vitamins, such as A, D, E, and K, do not dissolve in water but dissolve in fat and, therefore, require fat in the gut for their absorption. A layer of fat under the skin is a valuable insulator against internal heat loss. The layer of fat under the skin and that which surrounds organs is also an excellent shock absorber. Fat deposits in the back of the eyes serve as cushions when the head suffers a severe blow. During starvation, these deposits are lost, and the eyes become deep-set in the eye sockets, giving the person a ghostly appearance.
The pleasant taste and “mouth feel” of many foods is the result of fats. Their ingestion provides a full feeling after a meal because they tend to remain in the stomach so that it empties later than if fat were not present.
Kinds of Fats Important in Nutrition
Fats consist of the molecule of glycerol with three fatty acids attached. Several kinds of fats are commonly discussed with respect to nutrition (figure 25.3). Saturated fats have fatty acid portions that do not have any double bonds between carbons. They are generally of animal origin and are solids at room temperature. Unsaturated fats have double bonds between carbons in the fatty acid portion of the molecule. They are called oils because they are liquids at room temperature. They are generally of plant origin. Polyunsaturated fats have several double bonds in the fatty acid portion of the molecule. Trans fats are created when unsaturated fats are chemically altered (hydrogenated) to make them more solid. Although they are still unsaturated, they have fewer double bonds and have a slightly different structure from the fats normally produced by organisms. Trans fats cause an increase in the amount of “bad” lipids in the blood.

FIGURE 25.3. Saturated and Unsaturated Fats
Animal fats are saturated. Dairy products, meats, and foods cooked in animal fats are high in saturated fats. Plant fats are usually oils and are unsaturated fats.
Some fats contain the essential fatty acids, linoleic acid and linolenic acid, which cannot be synthesized by the human body and, therefore, must be a part of the diet. The body requires these essential fatty acids for normal growth, blood clotting, and healthy skin. Most diets that incorporate a variety of foods, including meats and vegetable oils, have enough of these essential fatty acids. A diet high in linoleic acid has also been shown to help reduce the amount of cholesterol in the blood. Because of negative health effects associated with saturated fats and trans fats in the diet, people are encouraged to reduce their total consumption of fats and to substitute unsaturated fats for saturated and trans fats.
How the Body Manages Fat
Body fat is produced when food consumption is higher than the amount needed for daily energy needs. This stored energy source can be called upon when a person does not consume enough Calories to meet daily energy demands. Early in the history of our species this was vitally important because obtaining food was often irregular. Periods of food scarcity were common and stored fat was important for survival. However, today food is abundantly available for most of us and often exceeds our daily needs. Thus, what once was an important mechanism for survival has become a problem of obesity for many people.
Proteins
Proteins are composed of amino acids linked together; however, not all proteins contain the same amino acids.
Functions of Proteins
Proteins are involved in a great number of structures and activities in the body. Cell membranes contain protein along with phospholipids. Structures like muscles, tendons, ligaments, skin, and connective tissues all have proteins as an important constituent. Many proteins are involved in regulating particular activities of the body. Enzymes control metabolism. Antibodies protect from disease. Hormones communicate. Many other proteins are involved in sending and receiving signals between cells. Proteins also provide a last-ditch source of energy during starvation when carbohydrate and fat reserves are used up. Thus, it is accurate to say that it is the proteins of an organism that determine its structure, metabolic abilities, and capacity to regulate and coordinate the various activities of the body.
Because proteins are so important, many people have a misconception about the amount of protein necessary in the diet. The amount is actually quite small (about 50 grams/day) and can be obtained easily. A hamburger, a half chicken breast, or a fish sandwich contains the daily amount of protein needed by most people.
Kinds of Proteins
From a nutritional point of view, proteins can be divided into two groups: complete proteins and incomplete proteins. Complete proteins contain all the amino acids required by the body and necessary for good health. Proteins derived from animal sources—meat, poultry, fish, eggs, milk—are complete proteins (figure 25.4). Incomplete proteins lack certain amino acids that the body must have to build essential proteins. Most plant proteins are deficient in one or more of the essential amino acids. For example, the amino acid lysine is absent or in very low quantities in wheat, rice, and corn and the amino acid tryptophan is limited in beans. However, eating a combination of beans and rice or corn and beans provides the equivalent of a complete protein.

FIGURE 25.4. Sources of Proteins
The muscle tissue of animals, eggs, and milk products are good sources of complete protein. Beans are also a good protein source but are an incomplete protein since they are low in the amino acid tryptophan.
The human body can manufacture some amino acids but is unable to manufacture others. Those the body cannot manufacture are called essential amino acids (table 25.1). Without adequate amounts of these essential amino acids in the diet, a person may develop a protein-deficiency disease.
TABLE 25.1. Sources of Essential Amino Acids
|
Essential Amino Acids |
Comments |
|
Threonine |
In most sources of protein |
|
Isoleucine |
In most sources of protein |
|
Methionine |
In most sources of protein |
|
Valine |
In most sources of protein |
|
Phenylalanine |
In most sources of protein |
|
Leucine |
In most sources of protein |
|
Tryptophan |
Deficient in legumes |
|
Lysine |
Deficient in grains |
|
Arginine |
Essential in infants only; in most sources of protein |
|
Histidine |
Essential in infants only; present in human and cow’s milk and infant formula |
In many parts of the world, people live on diets that provide the Calories they need from carbohydrates and fats but are low in complete protein. In part, this is because carbohydrates and fats are inexpensive to grow and process, in comparison with proteins. One protein-deficiency disease is kwashiorkor; its symptoms are easily seen (figure 25.5). A person with this deficiency has a distended belly, slow growth, and slow movement and is emotionally depressed. If the disease is caught in time, brain damage can be prevented and death averted. This requires a change in diet, including expensive protein, such as poultry, fish, beef, shrimp, or milk. As the world food problem increases, these expensive foods will be in even shorter supply and will become more and more costly.

FIGURE 25.5. Kwashiorkor
This starving child shows the symptoms of kwashiorkor, a protein- deficiency disease. If this child were treated with adequate protein containing all the amino acids, the symptoms could be reduced.
How the Body Manages Protein
Unlike carbohydrates and fats, proteins cannot be stored for later use. Because they are not stored and because they have many important functions, adequate amounts of protein must be present in the daily diet. However, a high-protein diet is not necessary. Only small amounts of protein—20 to 30 grams—are metabolized and lost from the body each day and must be replaced. A diet containing 50 grams of protein would easily cover this loss. Any protein in excess of that needed to rebuild lost molecules is metabolized to provide the body with energy.
Protein that makes up the structure of the body is protected from being metabolized to provide energy for cells. The mechanisms that protect protein are called proteinsparing mechanisms. During fasting or starvation, several kinds of metabolic adjustments allow the body to continue functioning without an input of food. Many of the body’s cells can use fat as their primary source of energy, thus protecting the more valuable protein. The breakdown of fats results in the production of compounds called ketones. Some of these ketones are released in the breath and can be detected as the odor of acetone. Acetone is an odor you would associate with fingernail polish. People who are fasting, anorexic, or diabetic or have other metabolic problems often have this “ketone breath.”
However, red blood cells and nervous tissue must have glucose, which can be supplied by the breakdown of glycogen stored in muscles and the liver. However, after a day or two of fasting, glycogen stores are depleted and glucose is unavailable from glycogen. Only at this point does the body begin to convert some of the amino acids from structural protein into glucose to supply these blood cells and nerve cells. During the early stages of starvation, the amount of fat in the body steadily decreases, but the amount of protein drops only slightly—20 to 30 grams per day (figure 25.6).
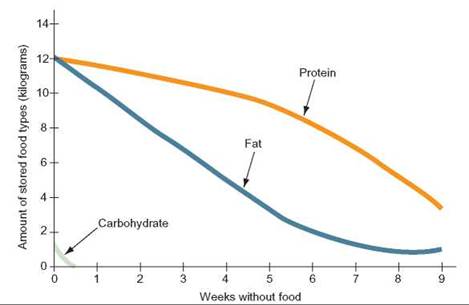
FIGURE 25.6. Protein-Sparing Mechanisms
The body uses various metabolic mechanisms to protect proteins during fasting or starvation. Notice that carbohydrate stores (glycogen) are depleted quickly and that fat stores fall much faster than protein. Protein-sparing mechanisms enable the body to protect essential enzymes and other proteins.
Although fat can supply energy for many cells during fasting or starvation, the fat cannot completely protect the proteins if there are no carbohydrates in the diet. With prolonged starvation, the fat stores are eventually depleted and structural proteins (as much as 125 grams per day) are used for all the body’s energy needs. When starvation reaches this point, it is usually fatal. People who are chronically undernourished do not have the protective effect of fat and experience the effects of starvation much more quickly than those who have stored fat. Children are particularly at risk, because they also need nutrients as building blocks for growth.
Vitamins
Vitamins are organic molecules needed in small amounts to maintain essential metabolic activities. Like essential amino acids and essential fatty acids, vitamins cannot be manufactured by the body. Table 25.2 lists vitamins for which there are recommended daily intakes.
TABLE 25.2. Sources and Functions of Vitamins
|
Name |
Recommended Daily Intake for Young Adults |
Physiological Value |
Readily Available Sources |
Other Information |
|
|
|
Women |
Men |
|
|
|
|
Water-Soluble Vitamins |
|||||
|
Vitamin B1 (thiamin) |
1.1 mg/d |
1.2 mg/d |
Maintains nerves and heart; involved in carbohydrate metabolism |
Whole grains, legumes, pork |
Larger amounts needed during pregnancy and lactation |
|
Vitamin B2 (riboflavin) |
1.1 mg/d |
1.3 mg/d |
Important in aerobic respiration reactions; maintains skin and mucous membranes |
Whole grains, dairy products, green vegetables; liver |
|
|
Vitamin B3 (niacin) |
14 mg/d |
16 mg/d |
Important in aerobic respiration reactions |
Whole grains, meat |
|
|
Vitamin B6 (pyridoxine pyridoxol, pyridoxamine) |
1.3 mg/d |
1.3 mg/d |
Builds red blood cells; maintains nervous system |
Whole grains, milk, meat, legumes, nuts, leafy green vegetables |
Large doses cause pain and numbness in the extremities |
|
Vitamin B12 (cobalamin) |
2.4 μg/d |
2.4 μg/d |
Protein and fat metabolism; forms red blood cells; maintains nervous system |
Animal foods only—dairy products, meat, poultry, seafood |
Stored in the liver; vegetarians must consume yeast or cereals with Bj2 added |
|
Vitamin C |
75 mg/d |
90 mg/d |
Antioxidant; maintains connective tissue |
Many fruits and vegetables; leafy green vegetables, tomatoes, potatoes |
|
|
Folate (folic acid) |
400 μg/d |
400 μg/d |
Coenzyme in metabolism; production of red blood cells |
Most foods, fortified cereals, beans |
Adequate amounts needed in pregnancy; low levels associated with neural tube defects |
|
Choline (lecithin) |
425 mg/d |
550 mg/d |
Component of cell membranes; component of acetylcholine—a neurotransmitter |
All foods |
Only important in people unable to consume food normally |
|
Fat-Soluble Vitamins |
|||||
|
Vitamin A |
700 μg/d |
900 μg/d |
Antioxidant; important in vision; maintains skin and intestinal lining |
Orange, red, and leafy green vegetables, liver |
Stored in liver; children have little stored; blindness results from lack of vitamin |
|
Vitamin D |
5 μg/d |
5 μg/d |
Needed to absorb calcium from gut; necessary for strong bones and teeth |
Vitamin D fortified milk; exposure of skin to sunlight |
Toxic in high concentrations |
|
Vitamin E |
15 mg/d |
15 mg/d |
Antioxidant; protects cell membranes |
Whole grains, nuts, vegetables, vegetable oils |
Only two cases of deficiency ever identified |
|
Vitamin K |
90 μg/d |
120 μg/d |
Blood clotting |
Leafy green vegetables |
Recommended for newborns |
Functions of Vitamins
Vitamins are involved as participants in many metabolic reactions. Some vitamins are actually incorporated into the structure of enzymes. Such vitamins are called coenzymes. For example, the B-complex vitamin niacin helps enzymes involved in aerobic cellular respiration. Several vitamins are antioxidants that protect cells. During normal metabolic processes, compounds called free radicals are produced. Free radicals are extremely reactive and can combine with and alter the structure of important molecules in the cell. Some of the vitamins (in particular, vitamins A, C, and E) combine with free radicals and neutralize their effects.
Kinds of Vitamins
From a nutritional point of view there are two kinds of vitamins: water-soluble vitamins and fat-soluble vitamins. The water-soluble vitamins are vitamin C and the various kinds of B vitamins. The fat-soluble vitamins are A, D, E, and K. Vitamin D deserves some special comment. Although vitamin D is found in certain foods, it is also formed when the ultraviolet light in sunshine strikes a cholesterol molecule in the skin, converting cholesterol to vitamin D. This means that vitamin D is not really a vitamin at all. It came to be known as a vitamin because of the mistaken idea that it was acquired only through food, rather than being formed in the skin on exposure to sunshine. It would be more correct to call vitamin D a hormone, but most people do not.
How Vitamins Are Managed in the Body
The water-soluble vitamins are not stored in the body and thus must be obtained in the diet on a daily basis because they are lost in the urine. Excess fat-soluble vitamins are stored in the liver and can be released for use when needed. Therefore, it is not necessary to have these vitamins in the diet every day.
Because many vitamins are inexpensive and their functions are poorly understood, many advocate large doses (megadoses) of vitamins to prevent a wide range of diseases. Often, the benefits advertised are based on fragmentary evidence and lack a clearly defined mechanism of action. The consumption of high doses of vitamins is unwise, because megadoses of many vitamins are toxic. For example, fat-soluble vitamins, such as vitamins A and D, are stored in body fat and the liver and can reach such high levels that ill health results. Excess vitamin A causes joint pain, hair loss, and jaundice. Excess vitamin D results in calcium deposits in the kidneys, high amounts of calcium in the blood, and bone pain. High doses of some of the water-soluble vitamins also have toxic effects: Vitamin B6 (pyridoxine) in high concentrations causes symptoms related to the nervous system, such as unsteady gait and numbness in the hands. However, inexpensive multivitamins that provide 100% of the recommended daily allowance can prevent or correct deficiencies caused by a poor diet without the danger of toxic consequences. Most people do not need vitamin supplements if they eat a well-balanced diet (How Science Works 25.2).
The lack of a particular vitamin in the diet can result in a vitamin-deficiency disease. Vitamin-deficiency diseases that show recognizable symptoms are extremely rare, except in countries with extreme food emergencies. Since vitamin A is necessary for vision, vitamin A deficiency is a leading cause of preventable blindness in children in developing countries. Night blindness is another manifestation of a lack of vitamin A.
HOW SCIENCE WORKS 25.2
Preventing Scurvy
Scurvy is a nutritional disease caused by a lack of vitamin C in the diet. Vitamin C is essential to the formation of collagen, a fiberlike protein important in most tissues. The symptoms of scurvy include the poor healing of wounds; fragile blood vessels, resulting in bleeding; a lack of bone growth; and a loosening of the teeth.
Scurvy is not common today; however, in the past, many people on long sea voyages developed scurvy because their diets lacked fresh fruits and vegetables. This was such a common problem that the disease was often called sea scurvy. Excerpts from a letter by a Dr. Harness to the First Lord of the Admiralty of the British navy describe the practice of using lemons to prevent scurvy on British ships: "During the blockade of Toulon in the summer of 1793, many of the ships' companies were afflicted with symptoms of scurvy; ... I was induced to propose ... the sending a vessel into port for the express purpose of obtaining lemons for the fleet; ... and the good effects of its use were so evident ... that an order was soon obtained from the commander in chief, that no ship under his lordship's command should leave port without being previously furnished with an ample supply of lemons. And to this circumstance becoming generally known may the use of lemon juice, the effectual means of subduing scurvy, while at sea, be traced."
A common term applied to British seamen during this time was limey.

Minerals
Minerals are elements found in nature that cannot be synthesized by the body. Table 25.3 lists the sources and functions of several common minerals. Because minerals are elements, they cannot be broken down or destroyed by metabolism or cooking. They commonly occur in many foods and in water.
TABLE 25.3. Sources and Functions of Minerals
|
Name |
Recommended Daily Intake for Young Adults |
Physiological Value |
Readily Available Sources |
Other Information |
|
|
|
Women |
Men |
|
|
|
|
Sodium |
Less than 1,500 mg/d |
Less than 1,500 mg/d |
Maintains cell membrane ionic balance; osmotic balance |
Present in most foods, table salt |
Most people get much more than needed; high levels associated with high blood pressure; people should restrict their intake to less than 1,500 mg/d |
|
Potassium |
4,700 mg/d |
4,700 mg/d |
Maintains cell membrane ionic balance |
Banana, legumes, potato skins, tomato |
Low amounts lead to acute nervous system, muscular system, and cardiac problems |
|
Calcium |
1,000 mg/d |
1,000 mg/d |
Builds bones and teeth; blood clotting; muscle contraction |
Dairy products, leafy green vegetables |
Children need 1,300 mg/d to support bone growth; vitamin D needed for calcium absorption |
|
Iron |
18 mg/d |
8 mg/d |
Necessary to make hemoglobin |
Meats, leafy green vegetables, seafood, legumes |
Because of menstruation women need more than men; pregnant women need twice the normal dose |
|
Phosphorus |
700 mg/d |
700 mg/d |
Maintains acid/base balance; enzyme cofactor; component of bones and teeth |
All foods |
Most people get more than needed; children need 1,250 mg/d to support bone growth |
|
Magnesium |
310 mg/d |
400 mg/d |
Coenzyme; necessary for bone mineralization; muscle and nerve function |
Leafy green vegetables, whole grains, legumes |
Found in chlorophyll |
|
Selenium |
55 μg/d |
55 μg/d |
Involved in many enzymatic reactions |
Meat, grains, seafood |
Toxic in high doses |
|
Zinc |
8 mg/d |
11 mg/d |
Involved in many enzymatic reactions; wound healing; fetal development |
Whole grains, beans, meat, fish, poultry |
Toxic in high doses |
|
Copper |
900 μg/d |
900 μg/d |
Involved in many enzymatic reactions; involved in iron metabolism |
Liver, poultry, shellfish, legumes, whole grains |
Toxic in high doses |
Functions of Minerals
Minerals retain their characteristics whether they are in foods or in the body, and each plays a different role in metabolism. Minerals can function as regulators, activators, transmitters, and controllers of various enzymatic reactions. For example, sodium (Na+) and potassium ions (K+) are involved in maintaining the polarity of cell membranes and are important in the transmission of nerve impulses, whereas magnesium ions (Mg++) facilitate energy release during reactions involving ATP. Without iron, not enough hemoglobin would be formed to transport oxygen, a condition called anemia, and a lack of calcium may result in osteoporosis. Osteoporosis is a calcium-deficiency disease in older adults that is tied to diet. Persons with this disease lose bone mass; their bones become more brittle and subject to fracture. The body needs calcium to maintain bone, so many adults take calcium supplements. However, calcium alone does not prevent osteoporosis. Bone strength is directly related to the amount of stress placed on the bone. Therefore, exercise is extremely important to assure that calcium will be incorporated into bones and improve their strength. Folic acid and other B vitamins may also help in preventing osteoporosis. Many minerals are important in the diet. In addition to those just mentioned, we need chlorine, cobalt, copper, iodine, phosphorus, sulfur, and zinc to remain healthy. With few exceptions, adequate amounts of minerals are obtained in a normal diet. Calcium and iron supplements may be necessary, however, particularly in women.
How the Body Manages Minerals
Many minerals such as sodium, potassium, and chloride are balanced by having excess amounts excreted in the urine. Minerals such as calcium and phosphorus are part of the structure of bone. These sources can be mobilized if the diet is low in these minerals, with the consequence of weakened bones. Pregnant and nursing mothers need to supplement their calcium intake to prevent bone loss. The body stores some iron bound to the protein ferritin in the liver, bone marrow, and spleen.
Water
Water is crucial to all life and plays many essential roles. Humans can survive weeks without food but would die in a matter of days without water. It is known as the universal solvent, because so many types of molecules are soluble in it. The human body is about 65% water. Even dense bone tissue consists of 33% water. All the chemical reactions in living things take place in water. It is the primary component of blood, lymph, and body tissue fluids. Inorganic and organic nutrients and waste molecules are also dissolved in water.
Dissolved inorganic ions, such as sodium (Na+), potassium (K+), and chloride (Cl-), are electrolytes because they form a solution capable of conducting electricity. The concentration of these ions in the body’s water must be regulated to prevent electrolyte imbalances.
Excesses of many types of waste are eliminated from the body dissolved in water; that is, they are excreted from the kidneys as urine or in small amounts from the lungs or skin through evaporation. In a similar manner, the evaporation of water from the skin cools the body. Water molecules are also essential reactants in all the various hydrolytic reactions of metabolism. Without it, the breakdown of molecules such as starch, proteins, and lipids would be impossible. With all these important roles played by water, it’s no wonder that nutritionists recommend that we drink the equivalent of at least eight glasses each day. This amount of water can be obtained from tap water, soft drinks, juices, and numerous food items, such as lettuce, cucumbers, tomatoes, and applesauce.
25.2. CONCEPT REVIEW
4. Why are some nutrients referred to as essential? Name them.
5. List the six main classes of nutrients as described by nutritionists. Describe the chemical nature of each and give one function of each class of nutrient.