CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
5. Enzymes, Coenzymes, and Energy
5.6. Enzymatic Reactions Used in Processing Energy and Matter
All living organisms require a constant supply of energy to sustain life. They obtain this energy through enzyme-controlled chemical reactions, which release the internal
potential energy stored in the chemical bonds of molecules (figure 5.10). Burning wood is a chemical reaction that results in the release of energy by breaking chemical bonds. The chemical bonds of cellulose are broken, and smaller end products of carbon dioxide (CO2) and water (H2O) are produced. There is less potential energy in the chemical bonds of carbon dioxide and water than in the complex organic cellulose molecules, and the excess energy is released as light and heat.
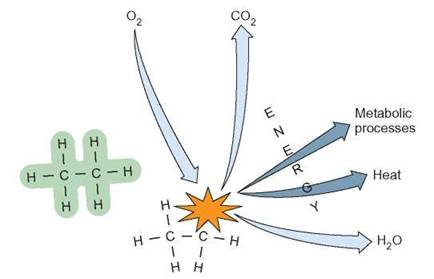
FIGURE 5.10. Life's Energy: Chemical Bonds
All living things use the energy contained in chemical bonds. As organisms break down molecules, they can use the energy released for metabolic processes, such as movement, growth, and reproduction. In all cases, there is a certain amount of heat released when chemical bonds are broken.
Biochemical Pathways
In living things, energy is also released but it is released in a series of small steps and each is controlled by a specific enzyme. Each step begins with a substrate, which is converted to a product, which in turn becomes the substrate for a different enzyme. Such a series of enzyme-controlled reactions is called a biochemical pathway, or a metabolic pathway. The processes of photosynthesis, respiration, protein synthesis, and many other cellular activities consist of a series of biochemical pathways. Biochemical pathways that result in the breakdown of compounds are generally referred to as catabolism. Biochemical pathways that result in the synthesis of new, larger compounds are known as anabolism. Figure 5.11 illustrates the nature of biochemical pathways.
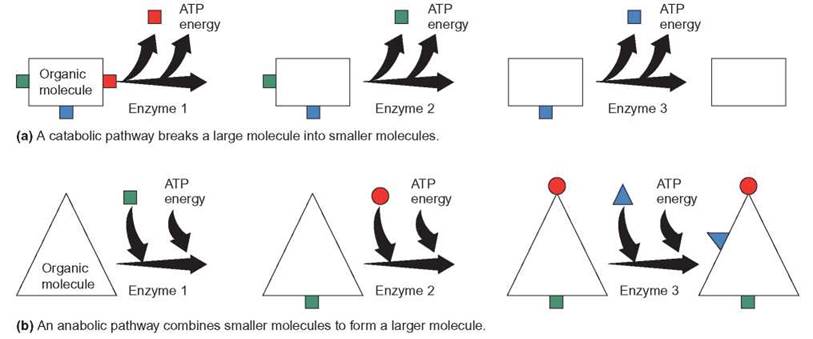
FIGURE 5.11. Biochemical Pathways
Biochemical pathways are the result of a series of enzyme-controlled reactions. In each step, a substrate is acted upon by an enzyme to produce a product. The product then becomes the substrate for the next enzyme in the chain of reactions. Such pathways can be used to break down molecules, build up molecules, release energy, and perform many other actions.
One of the amazing facts of nature is that most organisms use the same basic biochemical pathways. For example, the bacterium E. coli and human cells have an estimated 1,000 genes that are the same. These two drastically different cell types manufacture many of the same enzymes and, therefore, run many of the same pathways. However, because the kinds of enzymes an organism is able to produce depend on its genes, some variation occurs in the details of the biochemical pathways. The fact that so many kinds of organisms use essentially the same biochemical processes is a strong argument for the idea of evolution from a common ancestor. Once a successful biochemical strategy evolved, the genes and the pathways were retained (conserved) through evolutionary descendents, with slight modifications of the scheme.
Generating Energy in a Useful Form: ATP
The transfer of chemical energy within living things is handled by an RNA nucleotide known as adenosine triphosphate (ATP). Chemical energy is stored when ATP is made and is released when it is broken apart. An ATP molecule is composed of a molecule of adenine (a nitrogenous base), ribose (a sugar), and 3 phosphate groups (figure 5.12). If only 1 phosphate is present, the molecule is known as adenosine monophosphate (AMP).
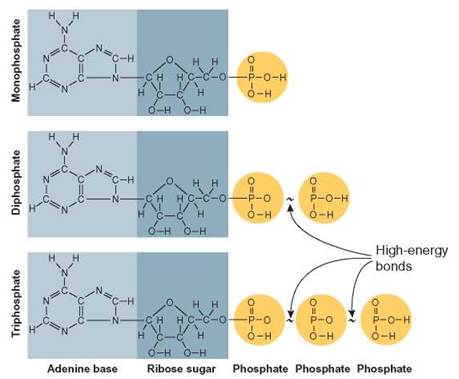
FIGURE 5.12. Adenosine Triphosphate (ATP)
An ATP molecule is an energy carrier. A molecule of ATP consists of several subunits: a molecule of adenine, a molecule of ribose, and 3 phosphate groups. The 2 end phosphate groups are bonded together by high-energy bonds. These bonds are broken easily, so they release a great amount of energy. Because they are high-energy bonds, they are represented by curved, solid lines.
When a second phosphate group is added to the AMP, a molecule of adenosine diphosphate (ADP) is formed. The ADP, with the addition of even more energy, is able to bond to a third phosphate group and form ATP. (Recall from chapter 3 that the addition of phosphate to a molecule is called a phosphorylation reaction.) The bonds holding the last 2 phosphates to the molecule are easily broken to release energy for cellular processes that require energy. Because the bond between these phosphates is so easy for a cell to use, it is called a high-energy phosphate bond. These bonds are often shown as solid, curved lines (-) in diagrams. Both ADP and ATP, because they contain high-energy bonds, are very unstable molecules and readily lose their phosphates. When this occurs, the energy held in the phosphate’s high-energy bonds can be transferred to a lower-energy molecule or released to the environment. Within a cell, specific enzymes (phosphorylases) speed this release of energy as ATP is broken down to ADP and P (phosphate). When the bond holding the third phosphate of an ATP molecule is broken, energy is released for use in other activities.
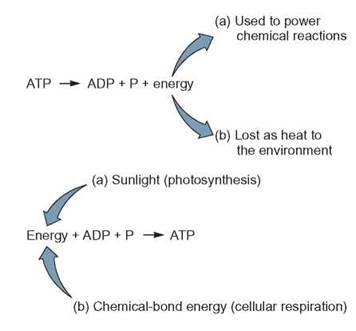
When energy is being harvested from a chemical reaction or another energy source, such as sunlight, it is stored when a phosphate is attached to an ADP to form ATP.
An analogy that might be helpful is to think of each ATP molecule used in the cell as a rechargeable battery. When the power has been drained, it can be recharged numerous times before it must be recycled (figure 5.13).
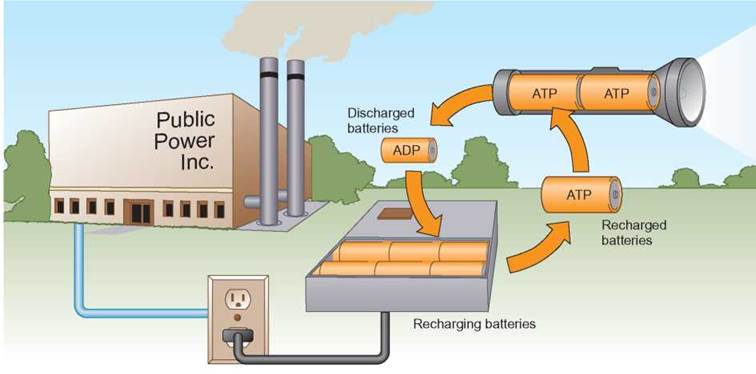
FIGURE 5.13. ATP: The Power Supply for Cells
When rechargeable batteries in a flashlight have been drained of their power, they can be recharged by placing them in a specially designed battery charger. This enables the right amount of power from a power plant to be packed into the batteries for reuse. Cells operate in much the same manner. When the cell’s “batteries,” ATPs are drained while powering a job, such as muscle contraction, the discharged “batteries,” ADPs can be recharged back to full ATP power.
Electron Transport
Another important concept that can be applied to many different biochemical pathways is the mechanism of electron transport. Because the electrons of an atom are on its exterior, the electrons in the outer energy level can be lost more easily to the surroundings, particularly if they receive additional energy and move to a higher energy level. When they fall back to their original position, they give up that energy. This activity takes place whenever electrons gain or lose energy. In living things, such energy changes are harnessed by special molecules that capture such “excited” electrons that can be transferred to other chemicals. These electron-transfer reactions are commonly called oxidation-reduction reactions. In oxidation-reduction (redox) reactions, the molecules losing electrons become oxidized and those gaining electrons become reduced. The molecule that loses the electron loses energy; the molecule that gains the electron gains energy.
There are many different electron acceptors or carriers in cells. However, the three most important are the coenzymes: nicotinamide adenine dinucleotide (NAD+), nicotinamide adenine dinucleotide phosphate (NADP+), and /lavin adenine dinucleotide (FAD). Recall that niacin is needed to make NAD+ and NADP+ and the riboflavin is needed to make FAD. Because NAD+, NADP+, FAD, and similar molecules accept and release electrons, they are often involved in oxidation-reduction reactions. When NAD+, NADP+, and FAD accept electrons, they become negatively charged. Thus, they readily pick up hydrogen ions (H+), so when they become reduced they are shown as NADH, NADPH, and FADH2. Therefore, it is also possible to think of these molecules as hydrogen carriers. In many biochemical pathways, there is a series of enzyme controlled oxidation-reduction reactions (electron-transport reactions) in which each step results in the transfer of a small amount of energy from a higher-energy molecule to a lower-energy molecule (figure 5.14). Thus, electron transport is often tied to the formation of ATP.
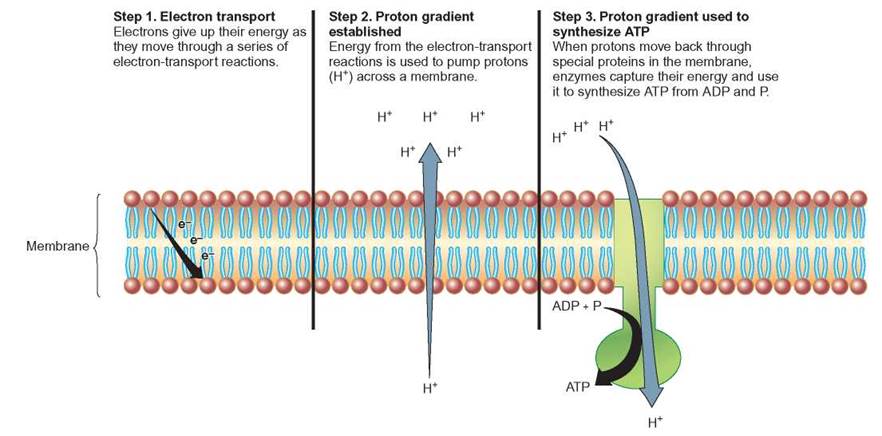
FIGURE 5.14. Electron Transport and Proton Gradient
The transport of high-energy electrons through a series of electron carriers can allow the energy to be released in discrete, manageable packets. In some cases, the energy given up is used to move or pump protons (H+) from one side of a membrane to the other and a proton concentration gradient is established. When the protons flow back through the membrane, enzymes in the membrane can capture energy and form ATP.
Proton Pump
In many of the oxidation-reduction reactions that take place in cells, the electrons that are transferred come from hydrogen atoms. A hydrogen nucleus (proton) is formed whenever electrons are stripped from hydrogen atoms. When these higher- energy electrons are transferred to lower-energy states, protons are often pumped across membranes. This creates a region with a high concentration of protons on one side of the membrane. Therefore, this process is referred to as a proton pump. The “pressure” created by this high concentration of protons is released when protons flow through pores in the membrane back to the side from which they were pumped. As they pass through the pores, an enzyme, ATP synthetase (a phosphorylase), uses their energy to speed the formation of an ATP molecule by bonding a phosphate to an ADP molecule. Thus, making a proton gradient is an important step in the production of much of the ATP produced in cells (review figure 5.14).
The four concepts of biochemical pathways, ATP production, electron transport, and the proton pump—are all interrelated. We will use these concepts to examine particular aspects of photosynthesis and respiration in chapters 6 and 7.
5.6. CONCEPT REVIEW
15. What is a biochemical pathway, and what does it have to do with enzymes?
16. Describe what happens during electron transport and what it has to do with a proton pump.
Summary
Enzymes are protein catalysts that speed up the rate of chemical reactions without any significant increase in the temperature. They do this by lowering activation energy. Enzymes have a very specific structure that matches the structure of particular substrate molecules. The substrate molecule comes in contact with only a specific part of the enzyme molecule— the attachment site. The active site of the enzyme is the place where the substrate molecule is changed. The enzyme-substrate complex reacts to form the end product. The protein nature of enzymes makes them sensitive to environmental conditions, such as temperature and pH, that change the structure of proteins. The number and kinds of enzymes are ultimately controlled by the genetic information of the cell. Other kinds of molecules, such as coenzymes, inhibitors, and competing enzymes, can influence specific enzymes. Changing conditions within the cell shift its enzymatic priorities by influencing the turnover number.
Enzymes are also used to speed and link chemical reactions into biochemical pathways. The energy currency of the cell, ATP, is produced by enzymatic pathways known as electron transport and proton pumping. The four concepts of biochemical pathways, ATP production, electron transport, and the proton pump are all interrelated.
Basic Review
1. Something that speeds the rate of a chemical reaction but is not used up in that reaction is called a
a. catalyst.
b. catabolic molecule.
c. coenzyme.
d. ATP.
2. The amount of energy it takes to get a chemical reaction going is known as
a. starting energy.
b. ATP.
c. activation energy.
d. denaturation.
e. Q.
3. A molecule that is acted upon by an enzyme is a
a. cofactor.
b. binding site.
c. vitamin.
d. substrate.
4. Your cells require _____ to manufacture certain coenzymes.
5. When a protein’s three-dimensional structure has been altered to the extent that it no longer functions, it has been
a. denatured.
b. killed.
c. anabolized.
d. competitively inhibited.
6. Whenever there are several different enzymes available to combine with a given substrate, _____ results.
7. In _____, a form of enzyme control, the end product inhibits one step of its formation when its concentration becomes high enough.
8. Which of the following contains the greatest amount of potential chemical-bond energy?
a. AMP
b. ADP
c. ATP
d. ARP
9. Electron-transfer reactions are commonly called _____ reactions.
10. As electrons pass through the pores of cell membranes, an enzyme, _____ (a phosphorylase), uses electron energy to speed the formation of an ATP molecule by bonding a phosphate to an ADP molecule.
11. If a cleaning agent contains an enzyme that will get out stains that are protein in nature, it can also be used to take out stains caused by oil. (T/F)
12. Keeping foods in the refrigerator helps make them last longer because the lower temperature _____ enzyme activity.
13. ATP is generated when hydrogen ions flow from a _____ to a _____ concentration after they have been pumped from one side of the membrane to the other.
14. What are teams competing for in a football game? _____
15. A person who is vitamin deficient will most likely experience a _____ in their metabolism.
Answers
1. a 2. c 3. d 4. vitamins 5. a 6. enzymatic competition 7. negative feedback 8. c 9. oxidation-reduction 10. ATP synthetase 11. F 12. slows/inhibits 13. higher, lower 14. the ball 15. Disruption
Thinking Critically
Nobel Prize Work
The following data were obtained by a number of Nobel Prize-winning scientists from Lower Slobovia. As a member of the group, interpret the data with respect to the following:
1. Enzyme activities
2. Movement of substrates into and out of the cell
3. Competition among various enzymes for the same substrate
4. Cell structure
Data
a. A lowering of the atmospheric temperature from 22°C to 18°C causes organisms to form a thick, protective coat.
b. Below 18°C, no additional coat material is produced.
c. If the cell is heated to 35°C and then cooled to 18°C, no coat is produced.
d. The coat consists of a complex carbohydrate.
e. The coat will form even if there is a low concentration of simple sugars in the surroundings.
f. If the cell needs energy for growth, no cell coats are produced at any temperature.