Must Know High School Biology - Kellie Ploeger Cox 2019
PART ONE Chemistry for Biology
Properties of Water
MUST ![]() KNOW
KNOW
![]() Water’s ability to hydrogen-bond gives it many unique and important qualities.
Water’s ability to hydrogen-bond gives it many unique and important qualities.
Water is really important. You know that, because you’ve probably heard that about 70% of Earth’s surface is covered with water, 60 % of your body is composed of water, and you need to drink plenty of water (otherwise you’ll become dehydrated!) What I want to talk about is why water is important on a molecular level. This little three-atom molecule is a key component of many life functions.
As we progress through this chapter, we will keep mentioning a special bond that occurs between water molecules. This bond (a hydrogen bond) is going to allow water to do many special things that contribute to its important function in biology.
A molecule of water (H2O) consists of two hydrogen atoms covalently bonded to a single oxygen atom. As we learned in Chapter 1, a covalent bond consists of two atoms sharing two electrons between them.
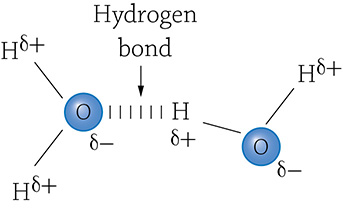
Hydrogen bonding between two water molecules
The figure above shows two water molecules involved in a specific type of intermolecular bond called a hydrogen bond. Each water molecule (circled) consists of two hydrogen atoms and an oxygen atom held together by covalent bonds (the straight lines). Because the covalent bonds are occurring within a single water molecule, they are examples of intramolecular bonds. Now, you notice those strange symbols (δ) next to the hydrogens and oxygen? That means “slightly negative” or “slightly positive.” Recall that a covalent bond involves the sharing of two electrons. The truth is, the atoms involves in that covalent bond don’t necessarily share equally. When a hydrogen and an oxygen participate in a covalent bond, the oxygen tends to pull the shared electrons closer to it, and away from the hydrogen. That gives the oxygen atom a slightly negative change; the electrons are hanging out closer to the oxygen atom. Because the negative charges are farther away from the hydrogen, the hydrogen atom is left with a slightly positive charge. The “slightly” adjective is really important because it is not a fully negative or positive charge…that would mean it was an ion (and it’s not because no electrons were fully lost or gained). Molecules that have this difference in charge distribution are called polar molecules.
The slightly negative oxygen of one water molecule is attracted to the slightly positive hydrogen of a different water molecule. It’s as if these two water molecules stick to each other like magnets, forming an intermolecular bond (remember that term from Chapter 1?). The reason all of this is important is because it relates to our must know concept. The ability of water molecules to form hydrogen bonds is way more important than you may think. Why will a closed bottle of water burst if left in the freezer (or even worse, why will water pipes in your house break if they freeze)? Why does a droplet of water make a dome-like shape? And why does it tend to be cooler along the coastline in the summer and a bit warmer in the winter? Read on for the answers to these mysteries!
Unique Properties of Water
Water is special. It is the liquid basis of all life. All living organisms are made mostly of water, and individual cells are about 80% water. Water has many unique properties that make life possible, including:
![]() Water is involved in cohesion and adhesion.
Water is involved in cohesion and adhesion.
![]() Water expands when freezes and becomes less dense.
Water expands when freezes and becomes less dense.
![]() Water has a high heat capacity.
Water has a high heat capacity.
Let’s go through each of these items in detail. As you do, keep in mind the must know idea that hydrogen bonding is the reason these things can occur.
![]() Water is involved in both cohesion and adhesion When a molecule of water hydrogen-bonds with itself, it is called cohesion. We learned about hydrogen bonds (and other intermolecular bonds) earlier in this chapter. Now, what we didn’t mention is that water can form hydrogen bonds with things other than water molecules; this is called adhesion. If you measured water in a graduated cylinder and noticed how the edge of the water sticks a bit to the glassware, you have seen an example of adhesion. The water is hydrogen-bonding to the glass (or plastic) of the graduated cylinder forming a meniscus. Cohesion and adhesion work together in one very important example we will visit again later in Chapter 20 (Plant Structure and Transport): it allows a “chain” of water molecules to be pulled up a plant’s vascular tissue.
Water is involved in both cohesion and adhesion When a molecule of water hydrogen-bonds with itself, it is called cohesion. We learned about hydrogen bonds (and other intermolecular bonds) earlier in this chapter. Now, what we didn’t mention is that water can form hydrogen bonds with things other than water molecules; this is called adhesion. If you measured water in a graduated cylinder and noticed how the edge of the water sticks a bit to the glassware, you have seen an example of adhesion. The water is hydrogen-bonding to the glass (or plastic) of the graduated cylinder forming a meniscus. Cohesion and adhesion work together in one very important example we will visit again later in Chapter 20 (Plant Structure and Transport): it allows a “chain” of water molecules to be pulled up a plant’s vascular tissue.
When water molecules hang around together, they act quite sticky. The surface of a body of water forms a “skin” of molecules hydrogen-bonded together. This gives water its tremendous surface tension. A rain droplet forms a lovely dome-shaped structure when it hits your car. Water striders can walk on water because the surface tension resists their tiny little legs punching through.
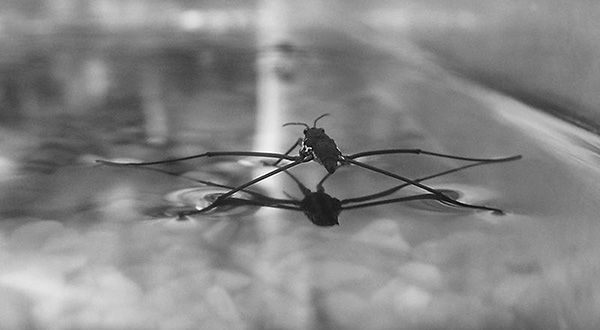
Water strider
Author: TimVickers. https://commons.wikimedia.org/wiki/File:Water_strider.jpg
![]()
IRL
It’s fun to have my students compete to see how many drops of water they can fit on a penny. The size of the water dome my students produce is shocking! After, I have them try to do the same thing with ethanol (which doesn’t have water’s spectacular surface tension) and they can only layer on a fraction of the droplets!
![]() Water expands when frozen and becomes less dense The hydrogen bonding that occurs causes the water molecules to align in such a way that water is less dense as a solid than as a liquid. That’s really weird! When water turns solid, the hydrogen-bonded molecules form what is called a “crystal lattice.” This arrangement gives the molecules quite a bit of elbow room. If you compare two equal volumes—one of liquid water and one of solid ice—there are fewer water molecules in the ice than the liquid! When water freezes, it expands and gets bigger—that’s why ice cubes sort of “pop” out of the ice cube tray. Furthermore, ice is less dense that its liquid counterpart…and that explains why those ice cubes float on the top of your drink and don’t sink to the bottom. This floating-ice fact has serious significance. First of all, in a large body of water (think oceans and lakes) floating ice insulates any water that’s below it. Secondly, if ice sank, those large bodies of water would easily freeze solid in the winter. Then, in the summer, only the top of the water would melt! How could anything live in lakes or oceans when their habitat freezes solid on a yearly basis? For that matter, would life exist AT ALL? The first forms of life evolved in the oceans. If they froze solid…NO LIFE. No us. Nothing. Whoa!
Water expands when frozen and becomes less dense The hydrogen bonding that occurs causes the water molecules to align in such a way that water is less dense as a solid than as a liquid. That’s really weird! When water turns solid, the hydrogen-bonded molecules form what is called a “crystal lattice.” This arrangement gives the molecules quite a bit of elbow room. If you compare two equal volumes—one of liquid water and one of solid ice—there are fewer water molecules in the ice than the liquid! When water freezes, it expands and gets bigger—that’s why ice cubes sort of “pop” out of the ice cube tray. Furthermore, ice is less dense that its liquid counterpart…and that explains why those ice cubes float on the top of your drink and don’t sink to the bottom. This floating-ice fact has serious significance. First of all, in a large body of water (think oceans and lakes) floating ice insulates any water that’s below it. Secondly, if ice sank, those large bodies of water would easily freeze solid in the winter. Then, in the summer, only the top of the water would melt! How could anything live in lakes or oceans when their habitat freezes solid on a yearly basis? For that matter, would life exist AT ALL? The first forms of life evolved in the oceans. If they froze solid…NO LIFE. No us. Nothing. Whoa!
Next time you swirl your ice cubes in your drink, ruminate on the significance the lowly ice cube has to the existence of life.
![]() Water has a high heat capacity. Take a moment to consider the three basic states of matter. In a solid, molecules pack the tightest; in a liquid, they are a little farther apart; and in a gas, they are floating freely, as far apart as the space allows. Water is weird because, as we just learned, they pack the tightest in the liquid form. But let us instead focus on going from a liquid to a gas. In order for any substance to evaporate (meaning it turns from a liquid to a gas), enough energy must be supplied to break the intermolecular bonds between the molecules. As we know, water molecules have quite a grip on each other, thanks to copious hydrogen bonding. In order to transition a liquid to a gas, those hydrogen bonds must be overcome (and broken apart) by heat. Consider a ring of water left on the table by your sweaty soda can. It will eventually absorb enough heat from the environment to evaporate and dry up. If it was instead a little puddle of another solvent, such as ethanol, hexane, or benzene, the liquid would evaporate and disappear in a fraction of the time that water took. So, what is the significance of this, since we’re learning biology right now? Water has the ability to stabilize temperatures because it resists temperature change, due to all that hydrogen bonding (our must know concept proving important once again). A large body of water will keep stable and resist wild swings in temperature. This is good for the life that lives in aquatic environments. Furthermore, because it resists a temperature change, oceans help to even out temperatures along the coastline—a bit warmer in the winter, and a bit cooler in the summer.
Water has a high heat capacity. Take a moment to consider the three basic states of matter. In a solid, molecules pack the tightest; in a liquid, they are a little farther apart; and in a gas, they are floating freely, as far apart as the space allows. Water is weird because, as we just learned, they pack the tightest in the liquid form. But let us instead focus on going from a liquid to a gas. In order for any substance to evaporate (meaning it turns from a liquid to a gas), enough energy must be supplied to break the intermolecular bonds between the molecules. As we know, water molecules have quite a grip on each other, thanks to copious hydrogen bonding. In order to transition a liquid to a gas, those hydrogen bonds must be overcome (and broken apart) by heat. Consider a ring of water left on the table by your sweaty soda can. It will eventually absorb enough heat from the environment to evaporate and dry up. If it was instead a little puddle of another solvent, such as ethanol, hexane, or benzene, the liquid would evaporate and disappear in a fraction of the time that water took. So, what is the significance of this, since we’re learning biology right now? Water has the ability to stabilize temperatures because it resists temperature change, due to all that hydrogen bonding (our must know concept proving important once again). A large body of water will keep stable and resist wild swings in temperature. This is good for the life that lives in aquatic environments. Furthermore, because it resists a temperature change, oceans help to even out temperatures along the coastline—a bit warmer in the winter, and a bit cooler in the summer.
REVIEW QUESTIONS
1. Draw two water molecules involved in a hydrogen bond. Label a covalent bond and a hydrogen bond.
2. When a water molecule forms a hydrogen bond with another water molecule, this is called ________________; when a water molecule hydrogen-bonds to another substance, it is called ________________.
3. What happens to water’s density when it freezes?
4. Water resists quick changes in temperature and doesn’t easily evaporate (or freeze); this is due to water’s ________________.
5. Why is it important to use the term partial when describing charges on a water molecule?
6. How does the polarity of a water molecule play a role in its ability to form hydrogen bonds?
7. What property of water is responsible for the creation of surface tension?
8. When water freezes, it becomes less dense and floats on the surface of bodies of water. Explain why this is helpful to aquatic life.
9. When water changes state—either from a solid to a liquid, or a liquid to a gas—what bonds are being broken?
