THE LIVING WORLD
Unit two. The Living Cell
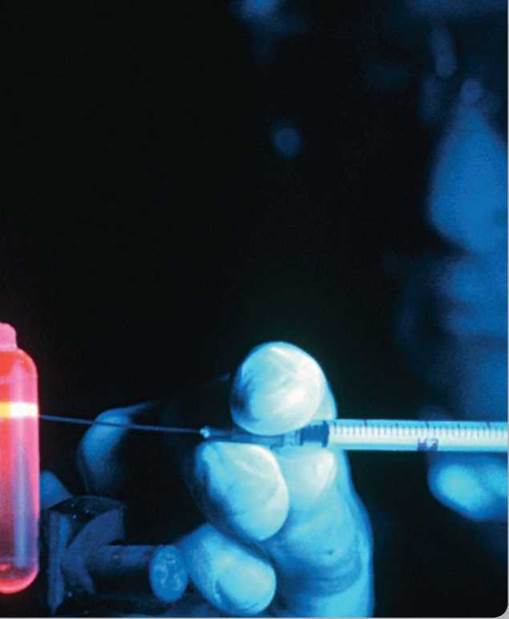
Using a syringe, this researcher is gently removing a glowing band of DNA, to be used in an experiment studying heredity. DNA, the carrier of an organism’s genes, is one of several kinds of very large molecules found in all organisms. A molecule is a collection of tiny atoms linked together. Atoms are the basic chemical elements. Only a few are found in any significant numbers in living things. An essential atom for life is carbon, which can assemble into DNA and other very large molecules. Interacting with water, these long carbon chains twist about each other, or fold up into compact masses. Much of the chemistry that goes on in organisms, determining what each individual is like, depends on the actions of large folded molecules called proteins. By promoting particular chemical reactions, proteins trigger the production of structural materials like carbohydrates and energy storage molecules like lipids. Because DNA encodes the information needed to assemble each protein present in an organism, it is the library of life.
3.1. Polymers Are Built of Monomers
The bodies of organisms contain thousands of different kinds of molecules and atoms. Organisms obtain many of these molecules from their surroundings and from what they consume. You might be familiar with some of the substances listed on nutritional labels, such as the one shown in figure 3.1. But what do the words on these labels mean? Some of them are names of minerals (atoms, see chapter 2), such as calcium and iron (also discussed in chapters 22, 24, 33, and others). Others are vitamins, which are discussed in later chapters (see chapter 25). Still others are the subject of this chapter: large molecules that make up the bodies of organisms and are found in our food, such as proteins, carbohydrates (including sugars), and lipids (including, fats, trans fats, saturated fats, and cholesterol). These molecules, called organic molecules, are formed by living organisms and consist of a carbon-based core with special groups attached. These groups of atoms have special chemical properties and are referred to as functional groups. Functional groups tend to act as units during chemical reactions and to confer specific chemical properties on the molecules that possess them. Five principal functional groups are listed in figure 3.2; the last column indicates the types of organic molecules that contain these functional groups.
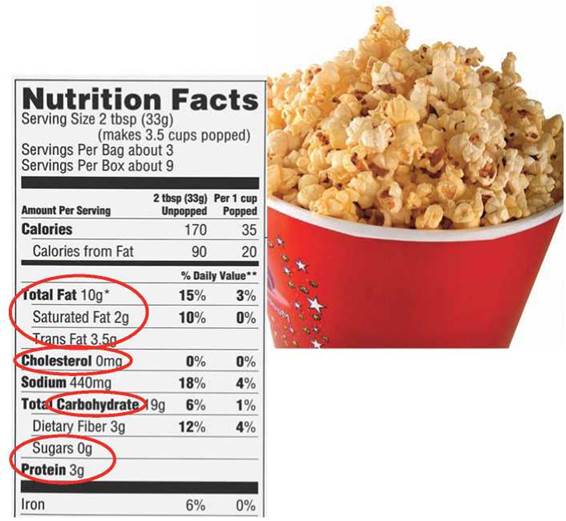
Figure 3.1. What's in a nutritional label?
Fats, cholesterol, carbohydrates, sugars, and proteins are just some of the molecules found in popcorn and discussed in this chapter.
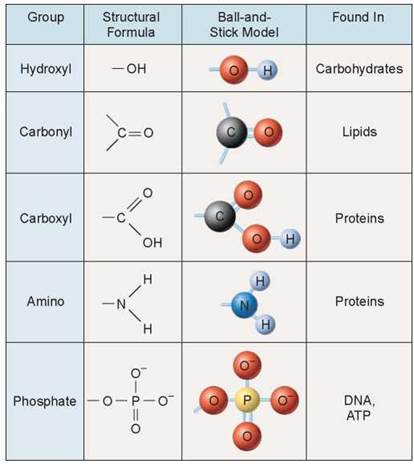
Figure 3.2. Five principal functional groups.
These functional groups can be transferred from one molecule to another and are common in organic molecules.
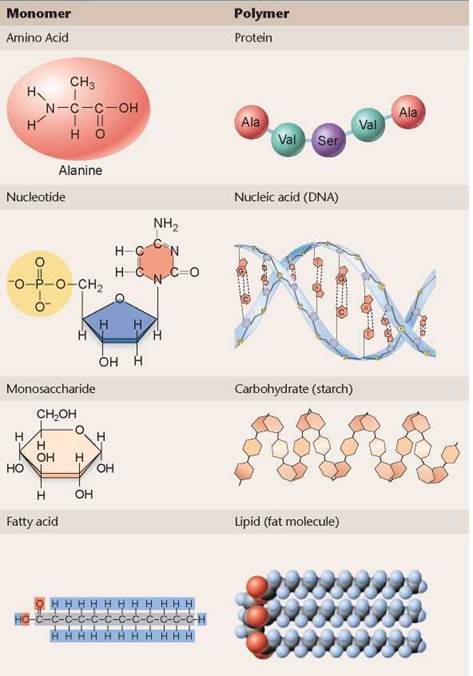
The bodies of organisms contain thousands of different kinds of organic molecules, but much of the body is made of just four kinds: proteins, nucleic acids, carbohydrates, and lipids. Called macromolecules because they can be very large, these four are the building materials of cells, the “bricks and mortar” that make up the bodies of cells and the machinery that runs within them.
The body’s macromolecules are assembled by sticking smaller bits, called monomers, together, much as a train is built by linking railcars together. A molecule built up of long chains of similar subunits is called a polymer. Table 3.1 lists the monomers in the first column that make up the polymers that are the basis for many cellular structures.
TABLE 3.1. MACROMOLECULES
Making (and Breaking) Macromolecules
The four different kinds of macromolecules (proteins, nucleic acids, carbohydrates, and lipids) are all put together in the same way: a covalent bond is formed between two subunits in which a hydroxyl group (OH) is removed from one subunit and a hydrogen (H) is removed from the other. This process (illustrated in figure 3.3a) is called dehydration synthesis because, in effect, the removal of the OH and H groups (highlighted by the blue oval) constitutes removal of a molecule of water—the word dehydration means “taking away water.” This process requires the help of a special class of proteins called enzymes to facilitate the positioning of the molecules so that the correct chemical bonds are stressed and broken. The process of tearing down a molecule, such as the protein or fat contained in food that is consumed, is essentially the reverse of dehydration synthesis: Instead of removing a water molecule, one is added. When a water molecule comes in, as shown in figure 3.3b, a hydrogen becomes attached to one subunit and a hydroxyl to another, and the covalent bond is broken. The breaking up of a polymer in this way is called hydrolysis.
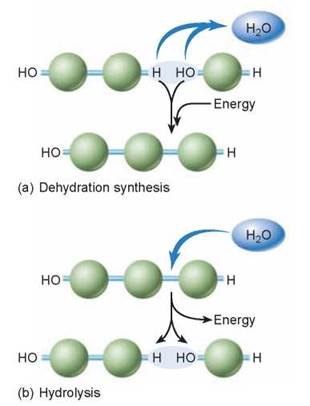
Figure 3.3. Dehydration and hydrolysis.
(a) Biological molecules are formed by linking subunits. The covalent bond between subunits is formed in dehydration synthesis, a process during which a water molecule is eliminated. (b) Breaking such a bond requires the addition of a water molecule, a reaction called hydrolysis.
Key Learning Outcome 3.1. Macromolecules are formed by linking subunits together into long chains, removing a water molecule as each link is formed. In contrast, macromolecules are broken down into their subunits by hydrolysis reactions, the addition of water molecules.