THE LIVING WORLD
Unit two. The Living Cell
3.4. Carbohydrates
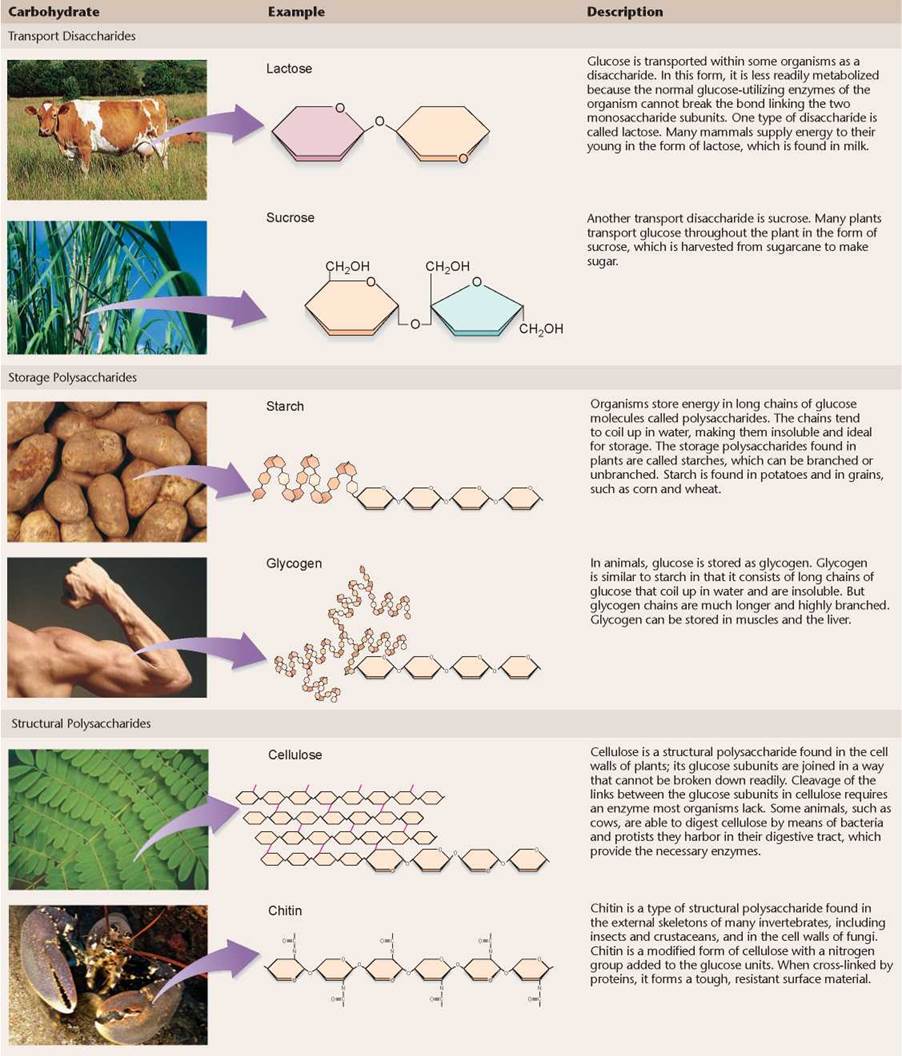
Polymers called carbohydrates make up the structural framework of cells and play a critical role in energy storage. A carbohydrate is any molecule that contains carbon, hydrogen, and oxygen in the ratio 1:2:1. Some carbohydrates are small monomers or dimers and are called simple carbohydrates. Others are long polymers and are called complex carbohydrates. Because they contain many carbon-hydrogen (C-H) bonds, carbohydrates are well-suited for energy storage. Such C-H bonds are the ones most often broken by organisms to obtain energy. Table 3.2 on the facing page shows some examples of carbohydrates.
TABLE 3.2. CARBOHYDRATES AND THEIR FUNCTIONS
Simple Carbohydrates
The simplest carbohydrates are the simple sugars or monosaccharides (from the Greek monos, single, and saccharon, sweet). These molecules consist of one subunit. For example, glucose, the sugar that carries energy to the cells of your body, is made of six carbons and has the chemical formula C6H12O6. A molecule of glucose is pictured in several ways in figure 3.12. The long chain of carbon atoms at the top of the figure is its formal chemical structure. When placed in water, the chain folds into the ring structure shown on the lower right. The individual atoms are depicted in the “3-D” space-filling model you see in the lower left. Another type of simple carbohydrate is a disaccharide, which forms when two monosaccharides link together through a dehydration reaction. In figure 3.13 you can see how the disaccharide sucrose (table sugar) is made by linking two six-carbon sugars together, a glucose (orange) and a fructose (green).
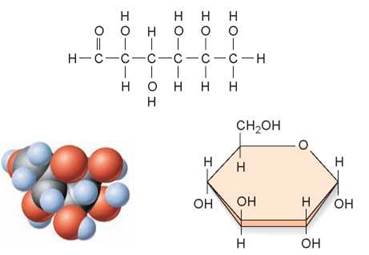
Figure 3.12. The structure of glucose.
Glucose is a monosaccharide and consists of a linear six-carbon molecule that forms a ring when added to water. This illustration shows three ways glucose can be represented diagrammatically.
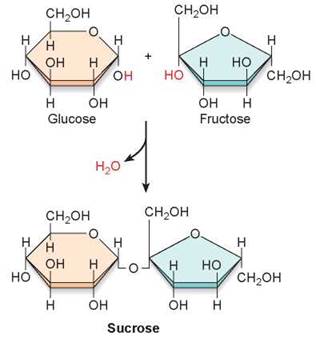
Figure 3.13 Formation of sucrose.
The disaccharide sucrose is formed from glucose and fructose in a dehydration reaction.
Complex Carbohydrates
Organisms store their metabolic energy by converting sugars, which are soluble, into insoluble forms that can be deposited in specific storage areas in the body. This trick is achieved by linking the sugars together into long polymer chains called polysaccharides. Plants and animals store energy in polysaccharides formed from glucose. The glucose polysaccharide that plants use to store energy is called starch—that is why potatoes are referred to as “starchy” food. In animals, energy is stored in glycogen, a highly insoluble macromolecule formed of glucose polysaccharides that are very long and highly branched. Plants and animals also use glucose chains as building materials, linking the subunits together in different orientations not recognized by most enzymes. These structural polysaccharides are chitin, in animals, and cellulose, in plants. The cellulose deposited in the cell walls of plant cells, like the cellulose strand shown in figure 3.14, cannot be digested by humans and makes up the fiber in our diets.
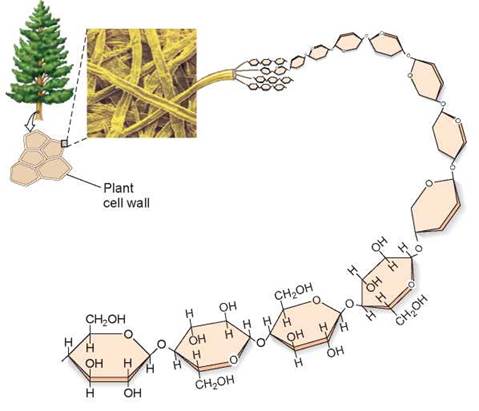
Figure 3.14. A polysaccharide: cellulose.
The polysaccharide cellulose is found in the cell walls of plant cells and is composed of glucose subunits.
Key Learning Outcome 3.4. Carbohydrates are molecules made of C, H, and O atoms. As sugars they store energy in C-H bonds, and as long polysaccharide chains they can provide structural support.