THE LIVING WORLD
Unit Six. Animal Life
27. How the Animal Body Defends Itself
27.6. B Cells: The Humoral Response
B cells also respond to helper T cells activated by interleukin-1. Like cytotoxic T cells, B cells have receptor proteins on their surfaces, a different receptor for each version of B cell. B cells recognize invading microbes much as cytotoxic T cells recognize infected cells, but unlike cytotoxic T cells, they do not go on the attack themselves. Rather, they mark the pathogen for destruction by mechanisms that have no “ID check” system of their own. Early in the immune response known as the humoral immune response, the markers placed by B cells alert complement proteins to attack the cells carrying them. Later in the response, the markers placed by B cells activate macrophages and natural killer cells.
The way B cells do their marking is simple and foolproof. Unlike the receptors on T cells, which bind only to antigen-MHC protein complexes on antigen-presenting cells, B cell receptors can bind to free, unprocessed antigens, shown in step 1 of figure 27.10. When a B cell encounters an antigen, antigen particles enter the B cell by endocytosis and get processed and placed on the surface complexed with MHC proteins. Helper T cells recognize the specific antigen, bind to the antigen-MHC protein complex on the B cell 2, and release interleukin-2, which stimulates the B cell to divide. In addition, free, unprocessed antigens stick to antibodies (the green Y-shaped structures) on the B cell surface. This antigen exposure triggers even more B cell proliferation. B cells divide to produce plasma cells that serve as short-lived antibody factories 3 and long-lived memory B cells 4 that remain in the body after the initial infection and are available to mount a quick attack if the antigen enters the body again.
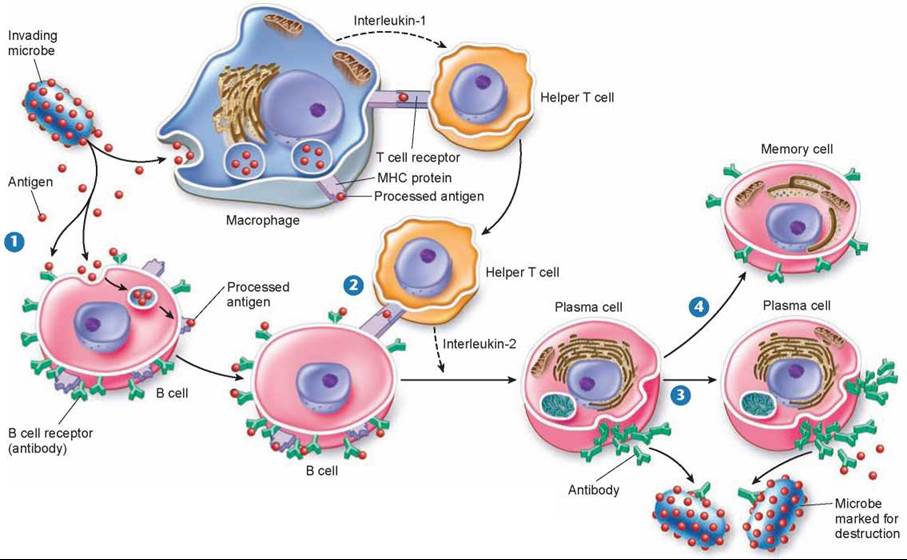
Figure 27.10. The B cell immune defense.
Invading particles are bound by B cells, which interact with helper T cells and are activated to divide. The multiplying B cells produce either memory B cells or plasma cells that secrete antibodies that bind to invading microbes and tag them for destruction by macrophages.
Antibodies are proteins in a class called immunoglobulins (abbreviated Ig), which is divided into subclasses based on the structures and functions of the antibodies. The five different immunoglobulin subclasses are as follows:
1. IgM. This is the first type of antibody to be secreted into the blood when the antigen is first encountered and to serve as a receptor on the B cell surface. These antibodies also promote agglutination reactions (causing antigen-containing particles to stick together, or agglutinate).
2. IgG. This is the major form of antibody secreted during a second or subsequent infection and the major one in the blood plasma.
3. IgD. These antibodies serve as receptors for antigens on the B cell surface. Their other functions are unknown.
4. IgA. This is the form of antibody in external secretions, such as saliva, mucus, and mother’s milk.
5. IgE. This form of antibody promotes the release of histamine and other agents that produce allergic symptoms, such as those of hay fever.
The plasma cells that are derived from B cells produce lots of the same particular antibody that was able to bind the antigen in the initial immune response. Flooding through the bloodstream, these antibody proteins (figure 27.11) are able to stick to antigens on any cells or microbes that present them, flagging those cells and microbes for destruction. Complement proteins, macrophages, or natural killer cells then destroy the antibody-displaying cells and microbes.
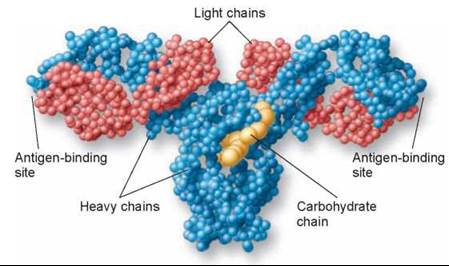
Figure 27.11. An antibody molecule.
In this molecular model of an antibody molecule, each amino acid is represented by a small sphere. Each molecule consists of four protein chains, two "light" (red) and two "heavy" (blue). The four protein chains wind around one another to form a Y shape. Foreign molecules, called antigens, bind to the arms of the Y.
The B cell defense is very powerful because it amplifies the reaction to an initial pathogen encounter a millionfold. It is also a very long-lived defense in that a few of the multiplying B cells do not become antibody producers. Instead they become a line of memory B cells that continue to patrol your body’s tissues, circulating through your blood and lymph for a long time—sometimes for the rest of your life.
Antibody Diversity
The vertebrate immune system is capable of recognizing as foreign practically any nonself molecule presented to it— literally millions of different antigens. Although vertebrate chromosomes contain only a few hundred receptor-encoding genes, it is estimated that human B cells can make between 106 and 109 different antibody molecules. How do vertebrates generate millions of different antigen receptors when their chromosomes contain only a few hundred versions of the genes encoding those receptors?
The answer to this question is that the millions of immune receptor genes do not exist as single sequences of nucleotides. Rather, they are assembled by stitching together three or four DNA segments that code for different parts of the receptor molecule. When an antibody is assembled, these different sequences of DNA are brought together to form a composite gene. The antibody molecule in figure 27.12 was produced by a composite gene: different sections of DNA were used to produce the constant regions (green), the joining regions (red), the diversity regions (blue), and the variable regions (yellow). This process is called somatic rearrangement.
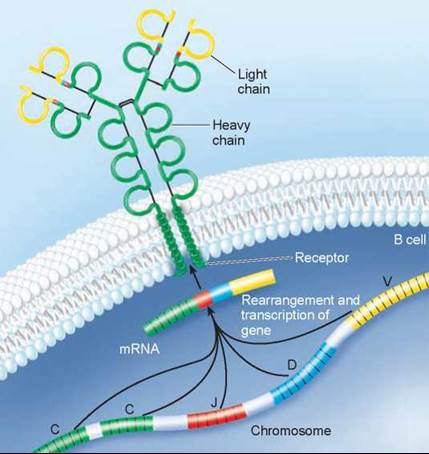
Figure 27.12. The antibody molecule is produced by a composite gene.
Different regions of the DNA code for different regions of the antibody (C, constant regions; J, joining regions; D, diversity regions; and V, variable regions) and are brought together to make a composite gene that codes for the antibody. By combining segments, an enormous number of different antibodies can be produced.
Two other processes generate even more sequences. First, the DNA segments are often joined together with one or two nucleotides off-register, shifting the reading frame during gene transcription and so generating a totally different sequence of amino acids in the protein. Second, random mistakes occur during successive DNA replications as the lymphocytes divide during clonal expansion. Both mutational processes produce changes in amino acid sequences, a phenomenon known as somatic mutation because it takes place in a somatic cell rather than in a gamete.
Because a cell may end up with any heavy chain gene and any light chain gene during its maturation, the total number of different antibodies possible is staggering: 16,000 heavy chain combinations x 1,200 light chain combinations = 19 million different possible antibodies. If one also takes into account the changes induced by somatic mutation, the total can exceed 200 million! It should be understood that although this discussion has centered on B cells and their receptors, the receptors on T cells are as diverse as those on B cells because they also are subject to similar somatic rearrangements and mutations.
Key Learning Outcome 27.6. In the humoral immune response, B cells label infecting and infected cells with antibodies to tag them for destruction by complement proteins, natural killer cells, and macrophages.