THE LIVING WORLD
Unit two. The Living Cell
5. Energy and Life
5.6. ATP: The Energy Currency of the Cell
Cells use energy to do all those things that require work, but how does the cell use energy from the sun or the potential energy stored in molecules to power its activities? The sun’s radiant energy and the energy stored in molecules are energy sources, but like money that is invested in stocks and bonds or real estate, these energy sources cannot be used directly to run a cell. To be useful, the energy from the sun or food molecules must first be converted to a source of energy that a cell can use, like someone converting stocks and bonds to ready cash. The “cash” molecule in the body is adenosine triphosphate (ATP). ATP is the energy currency of the cell.
Structure of the ATP Molecule
Each ATP molecule is composed of the three parts shown in figure 5.9: (1) a sugar (colored blue) serves as the backbone to which the other two parts are attached, (2) adenine (colored peach) is one of the four nitrogenous bases in DNA and RNA, and (3) a chain of three phosphates (colored yellow) contain high-energy bonds.
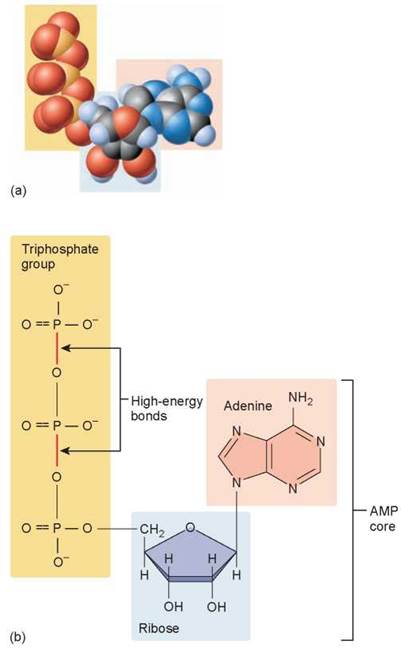
Figure 5.9. The parts of an ATP molecule.
The model (a) and structural diagram (b) both show that ATP consists of three phosphate groups attached to a ribose (five-carbon sugar) molecule. The ribose molecule is also attached to an adenine molecule (also one of the nitrogenous bases of DNA and RNA). When the endmost phosphate group is split off from the ATP molecule, considerable energy is released.
As you can see in the figure, the phosphates carry negative electrical charges, and so it takes considerable chemical energy to hold the line of three phosphates next to one another at the end of ATP. Like a coiled spring, the phosphates are poised to push apart. It is for this reason that the chemical bonds linking the phosphates are such chemically reactive bonds.
When the endmost phosphate is broken off an ATP molecule, a sizable packet of energy is released. The reaction converts ATP to adenosine diphosphate, ADP. The second phosphate group can also be removed, yielding additional energy and leaving adenosine monophosphate (AMP). Most energy exchanges in cells involve cleavage of only the outermost bond, converting ATP into ADP and Pi, inorganic phosphate:
![]()
Exergonic reactions require activation energy, and end- ergonic reactions require the input of even more energy, and so these reactions in the cell are usually coupled with the breaking of the phosphate bond in ATP, called coupled reactions. Because almost all chemical reactions in cells require less energy than is released by this reaction, ATP is able to power many of the cell’s activities, producing heat as a byproduct. Table 5.1 introduces you to some of the key cellular activities powered by the breakdown of ATP. ATP is continually recycled from ADP and Pi through the ATP-ADP cycle.
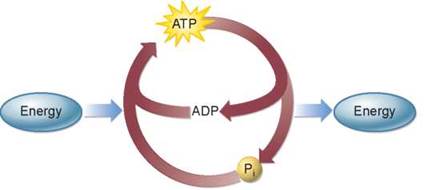
Cells use two different but complementary processes to convert energy from the sun and potential energy found in food molecules into ATP. Some cells convert energy from the sun into molecules of ATP through the process of photosynthesis, the subject of chapter 6. This ATP is then used to manufacture sugar molecules, converting the energy from ATP into potential energy stored in the bonds that hold the atoms together. All cells convert the potential energy found in food molecules into ATP through cellular respiration, the subject of chapter 7.
TABLE 5.1. HOW CELLS USE ATP ENERGY TO POWER CELLULAR WORK
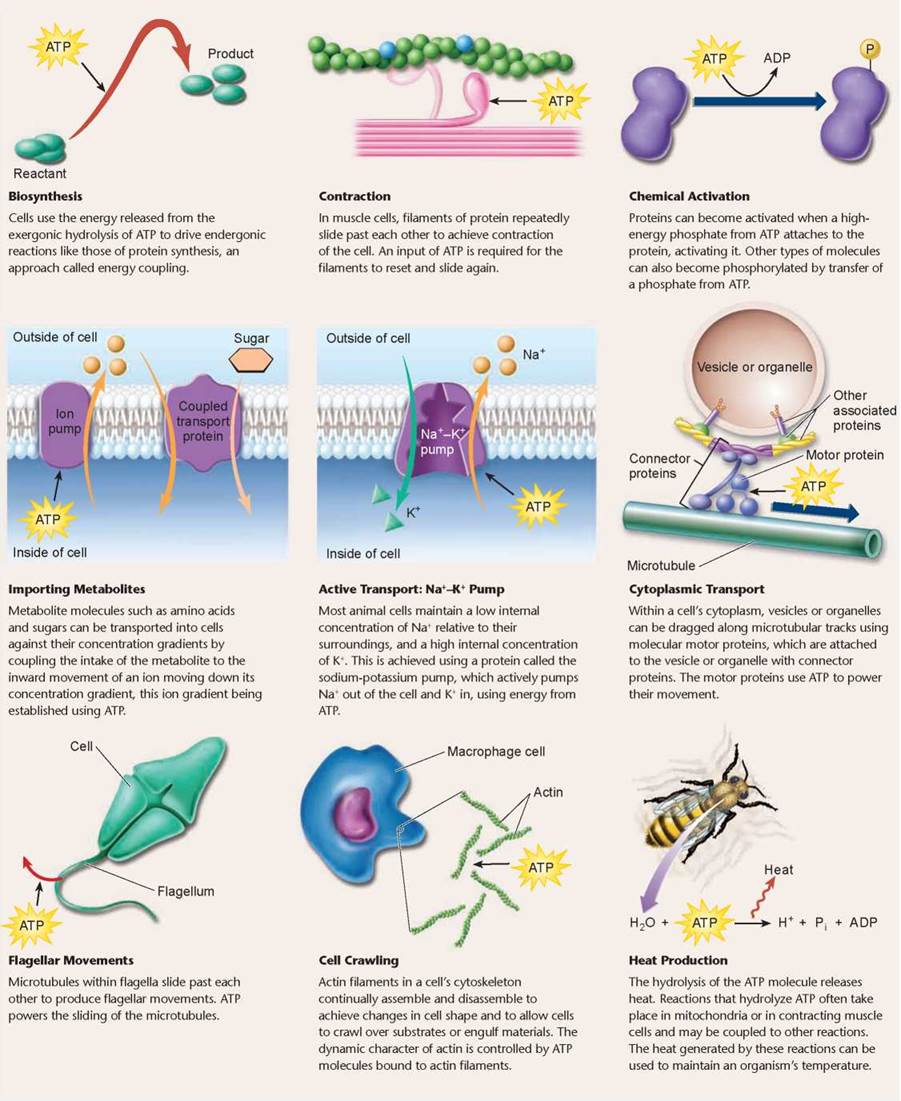
Key Learning Outcome 5.6. Cells use the energy in ATP molecules to drive chemical reactions.
Inquiry & Analysis
Do Enzymes Physically Attach to Their Substrates?
When scientists first began to examine the chemical activities of organisms, no one knew that biochemical reactions were catalyzed by enzymes. The first enzyme was discovered in 1833 by French chemist Anselme Payen. He was studying how beer is made from barley: First barley is pressed and gently heated so its starches break down into simple 2-sugar units; then yeasts convert these units into ethanol. Payen found that the initial breakdown requires a chemical factor that is not alive, and that does not seem to be used up during the process—a catalyst. He called this first enzyme diastase (we call it amylase today).
Did this catalyst operate at a distance, increasing reaction rate all around it, much as raising the temperature of nearby molecules might do? Or did it operate in physical contact, actually attaching to the molecules whose reaction it catalyzed (its "substrate”)?
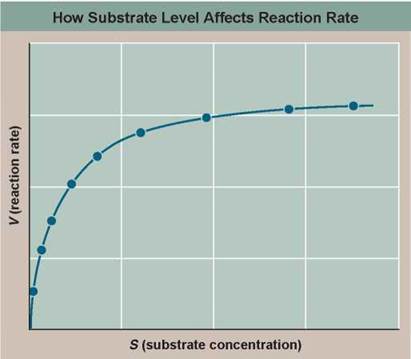
The answer was discovered in 1903 by French chemist Victor Henri. He saw that the hypothesis that an enzyme physically binds to its substrate makes a clear and testable prediction: In a solution of substrate and enzyme, there must be a maximum reaction rate, faster than which the reaction cannot proceed. When all the enzyme molecules are working full tilt, the reaction simply cannot go any faster, no matter how much more substrate you add to the solution. To test this prediction, Henri carried out the experiment whose results you see in the graph, measuring the reaction rate (V) of diastase at different substrate concentrations (S).
1. Making Inferences. As S increases, does V increase? If so, in what manner — steadily, or by smaller and smaller amounts? Is there a maximum reaction rate?
2. Drawing Conclusions. Does this result provide support for the hypothesis that an enzyme binds physically to its substrate? Explain. If the hypothesis were incorrect, what would you expect the graph to look like?
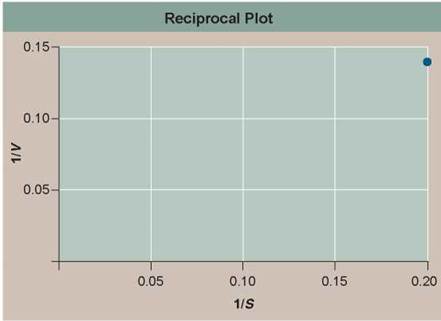
3. Further Analysis. If the smaller amounts by which V increases are strictly the result of fewer unoccupied enzymes being available at higher values of S, then the curve in Henri's experiment should show a pure exponential decline in V — mathematically, meaning a reciprocal plot (1/V versus 1/S) should be a straight line. If some other factor is also at work that reacts differently to substrate concentration, then the reciprocal plot would curve upward or downward. Fill in the reciprocal values in the table to the right, and then plot the values on the lower graph (1/S on the x axis and 1/V on the y axis). Is a reciprocal plot of Henri's data a straight line?
|
Trial |
S |
1/S |
V |
1/V |
|
1 |
5 |
0.200 |
7.7 |
0.130 |
|
2 |
10 |
|
15.4 |
|
|
3 |
25 |
|
23.1 |
|
|
4 |
50 |
|
30.8 |
|
|
5 |
75 |
|
38.5 |
|
|
6 |
125 |
|
40.7 |
|
|
7 |
200 |
|
46.2 |
|
|
8 |
275 |
|
47.7 |
|
|
9 |
350 |
|
48.5 |
|
Test Your Understanding
1. The ability to do work is the definition for
a. thermodynamics.
b. radiation.
c. energy.
d. entropy.
2. The first law of thermodynamics
a. says that energy recycles constantly, as organisms use and reuse it.
b. says that entropy, or disorder, continually increases in a closed system.
c. is a formula for measuring entropy.
d. says that energy can change forms, but cannot be made or destroyed.
3. The second law of thermodynamics
a. says that energy recycles constantly, as organisms use and reuse it.
b. says that entropy, or disorder, continually increases in a closed system.
c. is a formula for measuring entropy.
d. says that energy can change forms, but cannot be made or destroyed.
4. Chemical reactions that occur spontaneously are called
a. exergonic and release energy.
b. exergonic and their products contain more energy.
c. endergonic and release energy.
d. endergonic and their products contain more energy.
5. The catalysts that help an organism carry out needed chemical reactions are called
a. hormones.
b. enzymes.
c. reactants.
d. substrates.
6. Factors that affect the activity of an enzyme molecule include
a. peptides and energy.
b. thermodynamics.
c. temperature and pH.
d. entropy.
7. In order for an enzyme to work properly
a. it must have a particular shape.
b. the temperature must be within certain limits.
c. the pH must be within certain limits.
d. All of the above.
8. In competitive inhibition
a. an enzyme molecule has to compete with other enzyme molecules for the necessary substrate.
b. an enzyme molecule has to compete with other enzyme molecules for the necessary energy.
c. an inhibitor molecule competes with the substrate for the active site on the enzyme.
d. two different products compete for the same binding site on the enzyme.
9. Which of the following is not a component of ATP?
a. active site
b. ribose
c. adenine
d. phosphate groups
10. Endergonic reactions can occur in the cell because they are coupled with
a. the breaking of phosphate bonds in ATP.
b. uncatalyzed reactions.
c. activators.
d. all of the above.