THE LIVING WORLD
Unit Three. The Continuity of Life
8.5. Controlling the Cell Cycle
The events of the cell cycle are coordinated in much the same way in all eukaryotes. The control system that human cells use first evolved among the protists over a billion years ago; today, it operates in essentially the same way in fungi as it does in humans.
Check Points
The goal of controlling any cyclic process is to adjust the duration of the cycle to allow sufficient time for all events to occur. In principle, a variety of methods can achieve this goal. For example, an internal clock can be employed to allow adequate time for each phase of the cycle to be completed. This is how many organisms control their daily activity cycles. The disadvantage of using such a clock to control the cell cycle is that it is not very flexible. One way to achieve a more flexible and sensitive regulation of a cycle is simply to let the completion of each phase of the cycle trigger the beginning of the next phase, as a runner passing a baton starts the next leg in a relay race. Until recently, biologists thought this type of mechanism controlled the cell division cycle. However, we now know that eukaryotic cells employ a separate, centralized controller to regulate the process: At critical points in the cell cycle, further progress depends upon a central set of “go/no-go” switches that are regulated by feedback from the cell.
This mechanism is the same one engineers use to control many processes. For example, the furnace that heats a home in the winter typically goes through a daily heating cycle. When the daily cycle reaches the morning “turn on” checkpoint, sensors report whether the house temperature is below the set point (for example, 70°F). If it is, the thermostat triggers the furnace, which warms the house. If the house is already at least that warm, the thermostat does not start the furnace. Similarly, the cell cycle has key checkpoints where feedback signals from the cell about its size and the condition of its chromosomes can either trigger subsequent phases of the cycle or delay them to allow more time for the current phase to be completed.
Three principal checkpoints control the cell cycle in eukaryotes (figure 8.8):
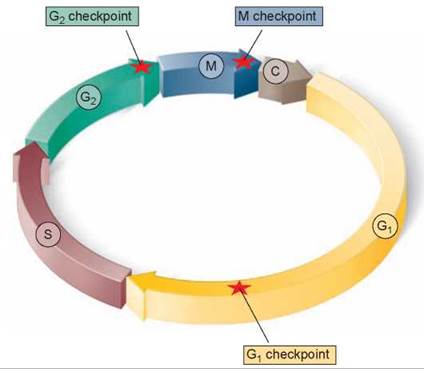
Figure 8.8. Control of the cell cycle.
Cells use a centralized control system to check whether proper conditions have been achieved before passing three key checkpoints in the cell cycle.
1. Cell growth is assessed at the G1 checkpoint. Located near the end of G1 and just before entry into S phase, the G1 checkpoint makes the key decision of whether the cell should divide, delay division, or enter a resting stage. In yeasts, where researchers first studied this checkpoint, it is called START. If conditions are favorable for division, the cell begins to copy its DNA, initiating S phase. The G1 checkpoint is where the more complex eukaryotes typically arrest the cell cycle if environmental conditions make cell division impossible or if the cell passes into an extended resting period called G0 (figure 8.9).
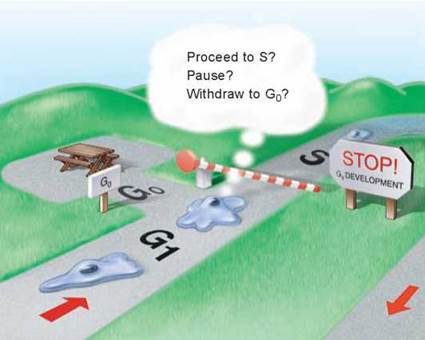
Figure 8.9. The G1 checkpoint.
Feedback from the cell determines whether the cell cycle will proceed to the S phase, pause, or withdraw into G0 for an extended rest period.
2. DNA replication is assessed at the G2 checkpoint. The second checkpoint, the G2 checkpoint, triggers the start of M phase. If this checkpoint is passed, the cell initiates the many molecular processes that signal the beginning of mitosis.
3. Mitosis is assessed at the M checkpoint. The third checkpoint, the M checkpoint, occurs at metaphase and triggers the exit from mitosis and cytokinesis and the beginning of G1.
Growth Factors Trigger Cell Division
Cell division is initiated by small proteins called growth factors. Growth factors work by binding to the plasma membrane and triggering intracellular signaling systems. Fibroblasts are cells that form connective tissue in the body and possess numerous receptors on their plasma membranes for one of the first growth factors to be identified: platelet- derived growth factor (PDGF). When PDGF binds to a membrane receptor, it initiates an amplifying chain of internal cell signals that stimulates cell division.
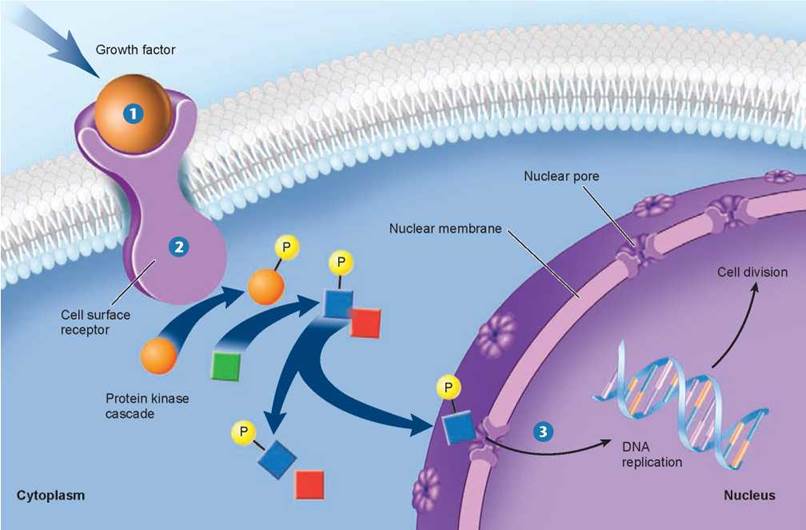
Figure 8.10. The cell proliferation-signaling pathway.
Binding of a growth factor sets in motion a cascading intracellular signaling pathway, which activates proteins in the nucleus that trigger cell division.
PDGF was discovered when investigators found that fibroblasts would grow and divide in tissue culture only if the growth medium contained blood serum (the liquid that remains after blood clots); blood plasma (blood from which the cells have been removed without clotting) would not work. The researchers hypothesized that platelets in the blood clots were releasing into the serum one or more factors required for fibroblast growth. Eventually, they isolated such a factor and named it PDGF.
Growth factors such as PDGF override cellular controls that otherwise inhibit cell division. When a tissue is injured, a blood clot forms and the release of PDGF triggers neighboring cells to divide, helping to heal the wound. Only a tiny amount of PDGF (approximately 10-10 M) is required to stimulate cell division.
Characteristics of Growth Factors. Over 50 different proteins that function as growth factors have been isolated, and more undoubtedly exist. A specific cell surface receptor “recognizes” each growth factor, its shape fitting that growth factor precisely. Figure 8.10 shows what happens when the growth factor 1 binds with its receptor 2. The receptor is activated and reacts by triggering a series of events within the cell, indicated by the arrows, that end with the replication of DNA and cell division 3. The cellular selectivity of a particular growth factor depends upon which target cells bear its unique receptor. Some growth factors, like PDGF and epidermal growth factor (EGF), affect a broad range of cell types, while others affect only specific types. For example, nerve growth factor (NGF) promotes the growth of certain classes of neurons, and erythropoietin triggers cell division in red blood cell precursors. Most animal cells need a combination of several different growth factors to overcome the various controls that inhibit cell division.
The G0 Phase. If cells are deprived of appropriate growth factors, they stop at the G1 checkpoint of the cell cycle. With their growth and division arrested, they remain in the G0 phase, as mentioned earlier. This nongrowing state is distinct from the interphase stages of the cell cycle, G1, S, and G2.
It is the ability to enter G0 that accounts for the incredible diversity seen in the length of the cell cycle among different tissues. Epithelial cells lining the gut divide more than twice a day, constantly renewing the lining of the digestive tract. By contrast, liver cells divide only once every year or two, spending most of their time in G0 phase. Mature neurons and muscle cells usually never leave G0.
Aging and the Cell Cycle
All humans die. However, while each of us knows we shall someday die, few of us can escape wishing we could delay the process. Some succeed. The oldest documented living person, Jeanne Calment of France, reached the age of 122 years in 1997. The tantalizing possibility of long life that she represents is one reason why there is such interest in the aging process; if we knew enough about it perhaps we could slow it. A wide variety of theories have been advanced to explain why we age. In recent years, scientists have come a long way toward unraveling the puzzle.
The first clue was the discovery that cells appear to die on schedule, as if following a script. In a famous experiment carried out in 1961, geneticist Leonard Hayflick demonstrated that fibroblast cells growing in tissue culture will divide only a certain number of times. As you can see in figure 8.11, after about 50 population doublings, cell division stops, the cell cycle blocked just before DNA replication. If a cell sample is frozen after the cell has undergone 20 doublings, when thawed the cell resumes growth for 30 more doublings, then stops.
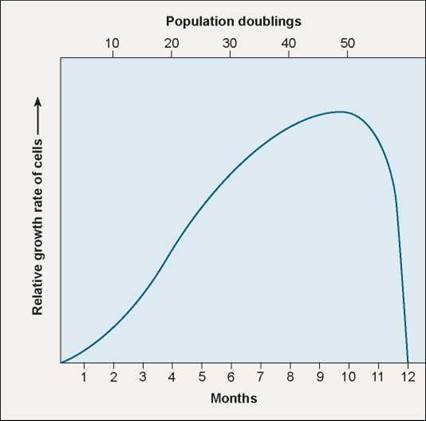
Figure 8.11. The Hayflick limit.
Normal human fibroblast (connective tissue) cells stop growing in culture after about 40 doublings, and within 10 more doublings all the cells are dead (blue line). When genetic engineers induce fibroblast cells to express the enzyme telomerase, proliferation continues long after 40 doublings.
An explanation of the “Hayflick limit” was suggested in 1978 when Elizabeth Blackburn of the University of California, San Francisco, first glimpsed an extra length of DNA at the ends of chromosomes. These telomeric regions, about 5,000 nucleotides long, are each composed of several thousand repeats of the sequence TTAGGG. Blackburn found the telomeric region to be substantially shorter in body tissue chromosomes than in those of germ-line cells, the egg and sperm. She speculated that in body cells a portion of the telomere cap was lost by a chromosome during each cycle of DNA replication.
Blackburn was right. The cell machinery that replicates the DNA of each chromosome sits on the last 100 units of DNA at the chromosome’s tip, and so cannot copy that bit. So each time the cell divides, its chromosomes get a little shorter. Eventually, after some 50 replication cycles, the protective telomeric cap is used up, and the cell line then enters senescence, no longer able to proliferate.
How do sperm and egg cells avoid this trap, dividing continuously for decades? Blackburn and collaborator Jack Szostak proposed that cells must possess a special enzyme that lengthens telomeres. In 1984 Blackburn’s graduate student Carol Greider found the enzyme, dubbed “telomerase.” Using it, egg and sperm maintain their chromosomes at a constant length of 5,000 nucleotide units. In body cells, by contrast, the telomerase gene is silent. For their discoveries, Blackburn, Greider, and Szostak received the 2009 Nobel Prize in Physiology or Medicine.
Later research has provided direct evidence for a causal relation between telomeric shortening and cell senescence. Using genetic engineering, teams of researchers from California and Texas in 1998 transferred into human body cell cultures a DNA fragment that unleashes each cell’s telomerase gene. The result was unequivocal. New telomeric caps were added to the chromosomes of the cells, and the cells with the artificially elongated telomeres did not senesce at the Hayflick limit, continuing to divide in a healthy and vigorous manner for more than 20 additional generations.
This research shows clearly that loss of telomere DNA eventually restrains the ability of human cells to proliferate. And yet every human cell possesses a copy of the telomerase gene that, if expressed, would rebuild the telomere. Why do our cells accept aging if they need not? The answer, it seems, is to avoid cancer. By limiting the number of divisions allotted to human cell lines, the body ensures that no cell can continue to divide indefinitely. Suppression of the telomerase gene is, in a very real sense, cancer suppression. When scientists examine cancer cells, they commonly find their telomerase genes have been activated and are maintaining telomeres at full length. Thus telomere shortening is a tumor-suppressing mechanism, one of your body’s key safeguards against cancer.
Key Learning Outcome 8.5. The complex cell cycle of eukaryotes is controlled with feedback at three checkpoints by protein signals called growth factors that initiate cell division. Telomeres play a key role in limiting cell proliferation.