Medical Microbiology
Section 2 The adversaries–host defences
11 The cellular basis of adaptive immune responses
Introduction
As we saw in a previous chapter, adaptive immune responses are generated by lymphocytes (Fig. 11.1), which are derived from stem cells differentiating within the primary lymphoid organs (bone marrow and thymus). From there, they colonize the secondary lymphoid tissues where they mediate the immune responses to antigens (Fig. 11.2). The lymph nodes are concerned with responses to antigens which are carried into them from the tissues, while the spleen is concerned primarily with antigens which reach it from the bloodstream (Fig. 11.3). Communication between these tissues and the rest of the body is maintained by a pool of recirculating lymphocytes which pass from the blood into the lymph nodes, spleen and other tissues and back to the blood by the major lymphatic channels such as the thoracic duct (Fig. 11.4). This traffic of lymphocytes between the tissues, the bloodstream and the lymph nodes enables antigen-sensitive cells to seek the antigen and to be recruited to sites at which a response is occurring. In addition, unencapsulated aggregates of lymphoid tissue termed ‘mucosa-associated lymphoid tissue’ or MALT, lie in the mucosal surface where they have the job of responding to antigens from the environment, particularly the heavy bacterial load in the intestine, by producing IgA antibodies for mucosal secretions. The lymphocytes which constitute the MALT system recirculate between these mucosal tissues using specialized homing receptors (Fig. 11.5).
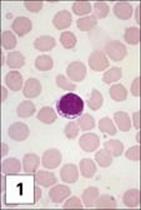
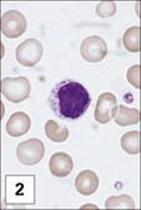
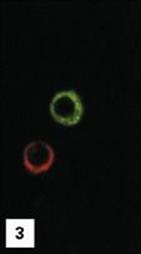
Figure 11.1 Lymphocytes and plasma cells. (1) Small B and T lymphocytes have a round nucleus and a high nuclear:cytoplasmic ratio. (2) A large granular lymphocyte with a lower nuclear:cytoplasmic ratio, an indented nucleus and azurophilic cytoplasmic granules. Fewer than 5% of T helper cells, and 30–50% of cytotoxic T cells, γδ T cells and natural killer (NK) cells have this morphology. (3) Antibody formed when B cells differentiate into plasma cells, here stained with fluoresceinated anti-human IgM (green) and rhodaminated anti-human IgG (red) showing extensive intracytoplasmic staining. Note that plasma cells produce only one class of antibody as the distinct staining reveals.
(1 and 2, stained with Giemsa, courtesy of A. Stevens and J. Lowe; 3, adapted from: Zucker-Franklin A. et al. (1988) Atlas of Blood Cells: Function and Pathology, 2nd edn, Vol. 11, Milan: EE Ermes; Philadelphia: Lea and Febiger.)
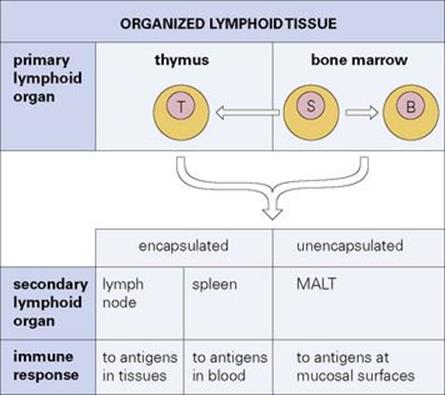
Figure 11.2 Organized lymphoid tissue. Stem cells (S) arising in the bone marrow differentiate into immunocompetent B and T cells in the primary lymphoid organs. These cells then colonize the secondary lymphoid tissues where immune responses are organized. MALT, mucosa-associated lymphoid tissue.
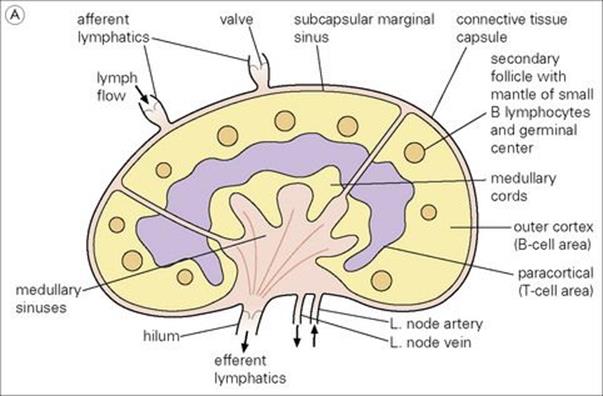
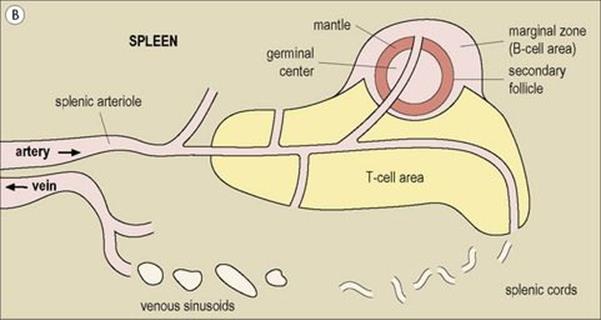
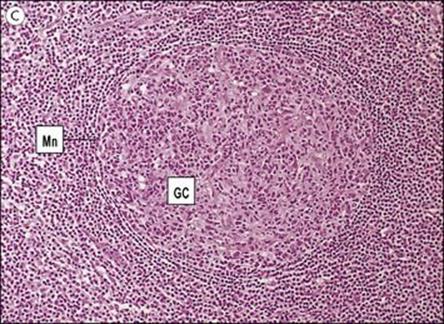
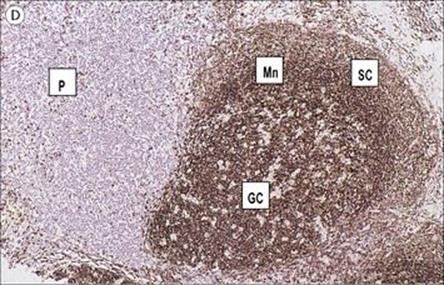


Figure 11.3 Structure of a lymph node and spleen. (A) Diagrammatic representation of section through a whole lymph node. The cortex is essentially a B-cell region where differentiation within the germinal centres of secondary follicles to antibody-forming plasma cells and memory cells occurs. (B) Diagrammatic representation of spleen showing B- and T-cell areas. (C) Structure of a secondary follicle. A large germinal centre (GC) is surrounded by the mantle zone (Mn). (D) Distribution of B cells in lymph node cortex. Immunochemical staining of B cells for surface immunoglobulin shows that they are concentrated largely in the secondary follicle, germinal centre (GC), mantle zone (Mn), and between the capsule and the follicle – the subcapsular zone (SC). A few B cells are seen in the paracortex (P), which contains mainly T cells. (E) Follicular dendritic cells in a secondary lymphoid follicle. This lymph node follicle is stained with enzyme-labelled monoclonal antibody to demonstrate follicular dendritic cells. (F) Germinal centre macrophages. Immunostaining for cathepsin D shows several macrophages localized in the germinal centre (GC) of a secondary follicle. These macrophages, which phagocytose apoptotic B cells, are called tingible body macrophages (TBM).
(Courtesy of A. Stevens and J. Lowe; C–F reproduced from Male D, Brostoff J, Roth DB, Roitt I. Immunology, 7th edition, 2006. Mosby Elsevier, with permission.)
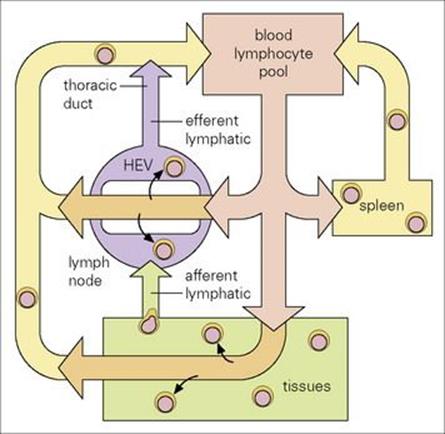
Figure 11.4 Lymphocyte traffic. The lymphocytes move through the circulation and enter the lymph nodes via the specialized endothelial cells of the postcapillary venules (HEVs). They leave through the efferent lymphatic vessels and pass through other nodes, finally entering the thoracic duct which empties into the circulation at the left subclavian vein (in humans). Lymphocytes enter the white pulp areas of the spleen in the marginal zones; they pass into the sinusoids of the red pulp and leave via the splenic vein.
(Adapted from: Roitt, I. M., Brostoff, J., Male, D. (2002) Immunology, 6th edn. London: Elsevier Science.)
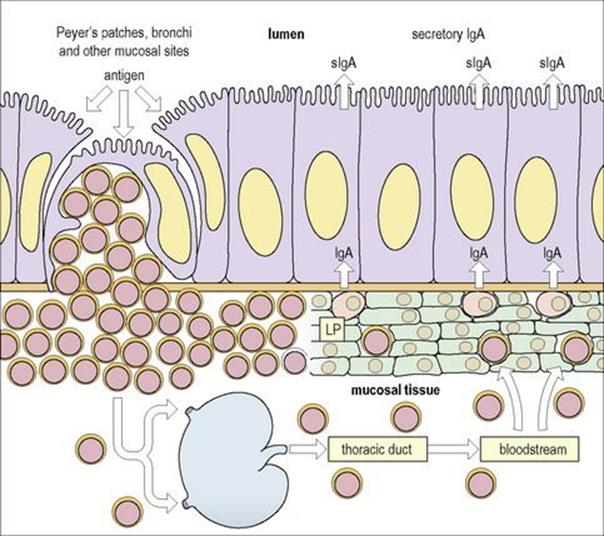
Figure 11.5 Mucosa-associated lymphoid tissue (MALT). Lymphoid cells which are stimulated by antigen in Peyer’s patches (or the bronchi or another mucosal site) migrate via the regional lymph nodes and thoracic duct into the bloodstream and thence to the lamina propria (LP) of the gut or other mucosal surfaces which might be close to or distant from the site of priming. Thus lymphocytes stimulated at one mucosal surface may become distributed selectively throughout the MALT system. This is mediated through specific adhesion molecules on the lymphocytes and the mucosal high-walled endothelium of the postcapillary venules.
(Adapted from: Roitt IM, Brostoff J, Male D. (2002) Immunology, 6th edn. London: Elsevier Science.)
B- and T-cell receptors
B and T cells can be distinguished by their surface markers
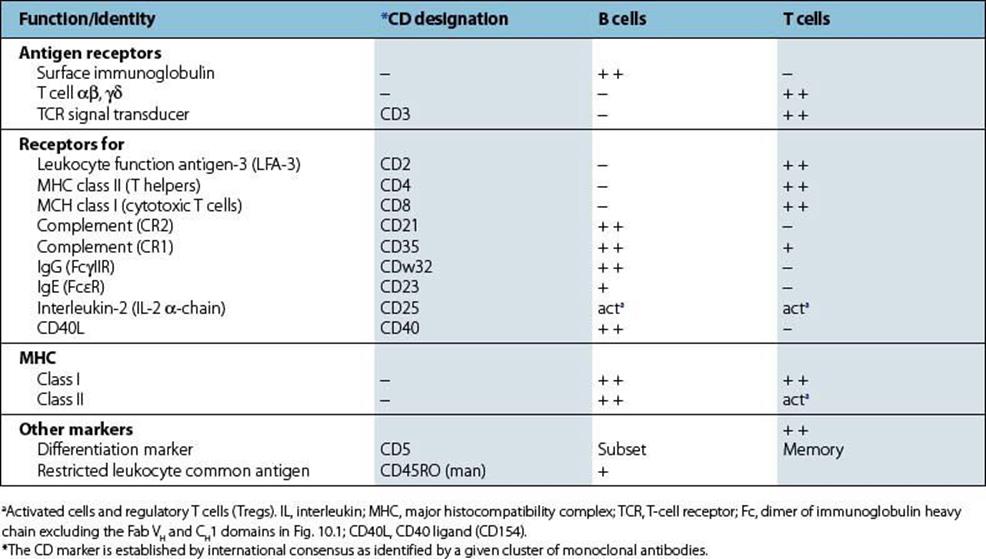
As they differentiate into populations with differing functions, B and T cells acquire molecules on their surface that reflect these specializations. It is possible to produce homogeneous antibodies of a single specificity, termed ‘monoclonal antibodies’, that can recognize such surface markers. When laboratories from all over the world compared the monoclonal antibodies they had raised, it was found that groups or clusters of monoclonal antibodies were each recognizing a common molecule on the surface of the lymphocyte. Each surface component so defined was referred to as a ‘CD’ molecule (Table 11.1), where CD refers to a ‘cluster of differentiation’.
Table 11.1 Surface markers on B and T cells
Each lymphocyte expresses an antigen receptor of unique specificity on its surface
Among the surface markers on the B and T cells referred to above are the receptors on the plasma membrane which are used to identify foreign antigens. B cells possess surface immunoglobulin, whereas the T-cell receptor (TCR) on the surface of the T lymphocyte acts as an antigen recognition unit (see Fig. 10.9). We now know that despite the very large number of different components that could be combined together in multiple ways to give a diversity of surface receptors, each B lymphocyte rearranges its germline genes coding for these receptors so that it selects one and only one of the specificities for each receptor polypeptide chain. It then expresses that receptor molecule on its surface (Fig. 11.6). Once this occurs, the other genes coding for these antigen receptors in the lymphocyte are no longer used. In other words, following this genetic rearrangement process, the lymphocyte becomes committed to the synthesis and expression of a single receptor type. An analogous process occurs in the rearrangement of the αβ and γδ genes coding for the TCR. Just as for B cells, each T cell expresses one and only one specific combination of receptor peptides, and therefore shows a single specificity to which it is committed for the whole of its lifespan.
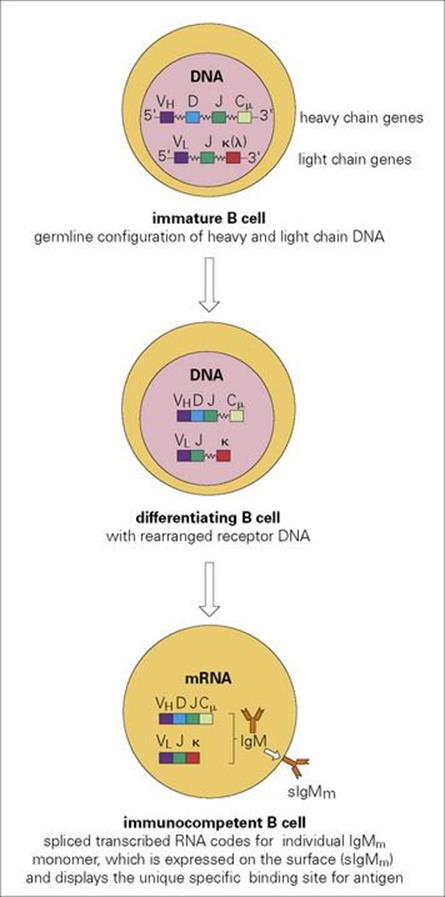
Figure 11.6 Differentiation events leading to the expression of unique IgM monomer sIgM on the surface of an immunocompetent B lymphocyte. There are of the order of 50 germline VH genes encoding the major portion of the variable region, with 25 minigenes encoding the D segment and six, the J region. As the cell differentiates, VH, D and J segments on one chromosome randomly fuse to generate lymphocytes with a very wide range of individual heavy chain variable domains. Variable region light chain domains are then formed by random VL to J recombination. Finally, the variable and constant region genes respectively recombine to encode a single antibody molecule which is expressed on the mature B-cell surface as an sIgM antigen receptor. When activated for antibody production, the transmembrane segment of IgM, which normally holds the molecule on the surface is spliced out at the RNA stage and the soluble form of the IgM is secreted. Subsequently, heavy chain constant region gene switch can occur to generate the various immunoglobulin classes, IgG, IgA, etc. Leader sequences have been omitted for simplicity.
Clonal expansion of lymphocytes
Antigen selects and clonally expands lymphocytes bearing complementary receptors. As there are such a large number of different possible specificities that lymphocytes can express, perhaps of the order of millions, there must of necessity be only a relatively small number of particular specificities to which lymphocytes are committed. Thus, when a microbe invades the body, the total number of lymphocytes initially committed to recognizing the antigens that go to make up a particular microbe is relatively small, and must be expanded to provide a sufficient number to protect the host. Evolution has provided a masterful solution to this problem. When a microbe enters the body, its component antigens combine with only those B lymphocytes whose surface receptors are complementary to the shape of these antigens. The B cells that bind the antigen become activated and proliferate clonally under the influence of soluble growth factors termed cytokines (see section on Cytokines below) to form a large population of cells derived from the original (Fig. 11.7). The majority of these events occur within the lymphoid structure known as a germinal centre (see Fig. 11.3).
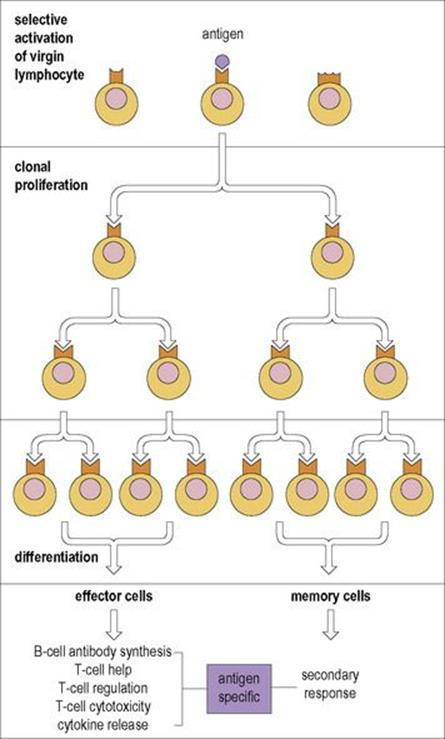
Figure 11.7 Generation of a large population of effector and memory cells by clonal proliferation after primary contact of B or T cell with antigen. A fraction of the progeny of the original antigen-reactive lymphocytes become non-dividing memory cells, whereas the others become the effector cells of humoral or cell-mediated immunity. Memory cells require fewer cycles before they develop into effectors, thus shortening the reaction time for the secondary response.
In the case of B cells, a large proportion of the clonally expanded lymphocytes become plasma cells (see Fig. 11.1), dedicated to the synthesis and secretion of antibodies. Since these plasma cells are derived from a parent cell that is already committed to the production of only one specific antibody, the final product is identical to the molecule that was posted on the surface of the original antigen-recognizing cell. Or at least almost so, because somatic mutation of the lymphocytes within the germinal centres which are synthesizing this antibody fine tunes the binding efficiency of the eventual product. The net result is that we have the production of large amounts of antibody which, like that on the surface of the parent cell, can combine with the invading antigen (Fig. 11.7).
A similar process of clonal selection and expansion occurs with T cells, producing a large number of T-cell effectors with the same specificity as the original parent cell; some of these cells release cytokines, whereas others have cytotoxic functions so that they act as effectors of T-cell-mediated immunity. One difference between T and B cells is that the T-cell receptors do not undergo further selection as a result of somatic mutation. Of crucial significance is the fact that in the case of both B and T cells, a fraction of the clonally expanded population differentiates into resting memory cells (Fig. 11.7). Thus, more cells are capable of recognizing the microbial antigen in any subsequent infection than in the initial virgin population that existed before the primary infection occurred. Human memory T cells can be identified by surface markers such as CD45RO, while memory B cells express CD27 and surface IgG, IgA or IgE.
The role of memory cells
Vaccination depends upon secondary immune responses being bigger and brisker than primary responses
In general, memory cells, as compared with naive cells, are more readily stimulated by a given dose of antigen. This occurs because they have greater combining power, in the case of B cells through mutation and selection during the primary response, and for T cells, which do not undergo affinity maturation, through increased expression of accessory adhesion molecules, CD2, LFA-1, LFA-3 and intercellular adhesion molecule-1 (ICAM-1), which enable the lymphocyte to bind more strongly to the specialized cells which present antigen. These factors, combined with the increased number of lymphocytes specific for a given antigen present in the memory pool produced by the primary response, result in a much stronger antibody or T-cell response on second contact with antigen. This provides the principle for vaccination (Fig. 11.8). The microbe or antigen to be used for vaccination is modified in such a way that it no longer produces disease or damage, but still retains the majority of its antigenic shapes. The primary response produced by the vaccination gives rise to a pool of memory cells, which can generate an abundant secondary response on subsequent contact with the antigen during a natural infection. Memory is usually long-lived, often extending over many years. There are many possible reasons for this: memory cells themselves may be innately long-lived or they may be sustained by gentle proliferation through subsequent contact with antigen present in reservoirs within the body or introduced by subclinical infection. An alternative mechanism in the case of T cells may be through stimulation by the cytokine IL-15 and in the case of B cells by anti-idiotypes (anti-antibodies produced in response to the combining region of the first antibody which may stimulate the memory B cells by ‘tweaking’ their surface receptors).
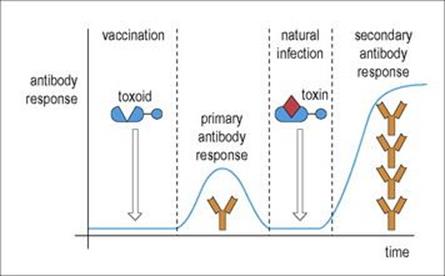
Figure 11.8 Primary and secondary responses. The antibody response on the second contact with antigen is more rapid and more intense. Therefore, following vaccination with a benign form of the antigen (in the example shown, a chemically modified form of tetanus toxin where the toxic element has been destroyed) to produce a primary response, subsequent contact with antigen in the form of a natural infection evokes the more efficient secondary response.
Stimulation of lymphocytes
T lymphocytes are activated by antigen presented on specialized cells
Naive T cells are potently stimulated by interdigitating dendritic cells (IDC), which are specialized antigen-presenting cells (APCs). Immature IDCs in the tissues take up antigen which is then processed and presented on the surface as a peptide complexed with MHC class II. The IDC migrates to the T-cell region of the draining lymph node, where it stimulates several T lymphocytes with which it makes contact through recognition of the MHC-peptide complex by the specific T-cell receptor, and by accessory interaction of the B7 co-stimulator with surface CD28 and by production of various cytokines which control the differentiation of distinct T-cell subsets (Fig. 11.9).
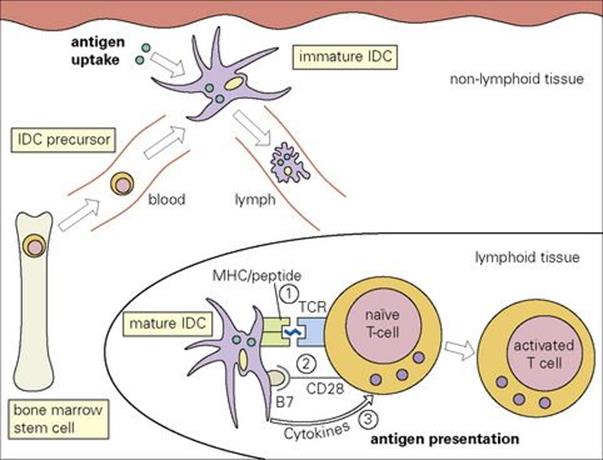
Figure 11.9 Migration and maturation of interdigitating dendritic cells (IDC). The precursors of the IDCs are derived from bone marrow stem cells. They travel via the blood to non-lymphoid tissues. These immature IDCs, e.g. Langerhans cells in skin, are specialized for antigen uptake. Subsequently, they travel via the afferent lymphatics to take up residence within secondary lymphoid tissues, where they express high levels of MHC class II and co-stimulatory molecules such as B7. These cells are highly specialized for the activation and differentiation of naive T cells which are effected through three signals: (1) TCR binding to MHC-peptide complex, (2) B7–CD28 co-stimulation and (3) cytokine release.
(Reproduced with minor additions with permission from: Roitt, I. M. and Delves, P. J. (2001) Roitt’s Essential Immunology, 10th edn. Oxford: Blackwell Science.)
As noted above, primed T cells are more readily stimulated by antigen than naive cells, and in this case, macrophages can function as the antigen-presenting cell.
Some antigens stimulate B cells without the need for intervention by T lymphocytes
These so-called T-independent antigens are of two main types:
1. Antigens of the first type contain molecular features that enable them to stimulate a wide variety of B cells independently of their specific antigen receptors; they are therefore referred to as ‘polyclonal activators’. Those B cells carrying surface receptors that recognize epitopes on the polyclonal activator attract the molecule to their surfaces and are preferentially stimulated relative to the remainder of the B-cell population (Fig. 11.10).
2. The second type of T-independent antigen involves repeating determinants presented on the surface of specialized macrophages located within the marginal zone associated with splenic secondary follicles with germinal centres or within the lymph node subcapsular sinus (see Fig. 11.3A, B), which can cross-link immunoglobulin receptors on the B cell and apparently stimulate the lymphocyte directly (Fig. 11.10).

Figure 11.10 B-cell activation by T-independent antigens. The requirements for an antigen-presenting cell (APC) for type 2 antigens are still uncertain. Ig, immunoglobulin.
One feature of both these types of T-independent antigen is that they give rise mainly to low-affinity IgM rather than IgG antibody responses and rarely induce a memory response.
Antibody production frequently requires T-cell help
The majority of antigens will stimulate B cells only if they have the assistance of T-lymphocyte helper (Th) cells. The sequence of events is as follows:
• In stage 1, the antigen is processed by an antigen-presenting cell and primes a Th cell with a complementary receptor on its surface as described above.
• In stage 2, a B cell with surface receptors complementary to an epitope on the original antigen captures the antigen on its receptor, internalizes it and, after processing, also presents a derived peptide on its surface in association with endogenous MHC class II molecules. This is the complex against which the Th cell was originally primed, and recognition of the processed antigen by the primed Th cell and of CD40 by the co-stimulatory CD40L causes activation of the B cell, with subsequent activation, proliferation and maturation (Fig. 11.11).
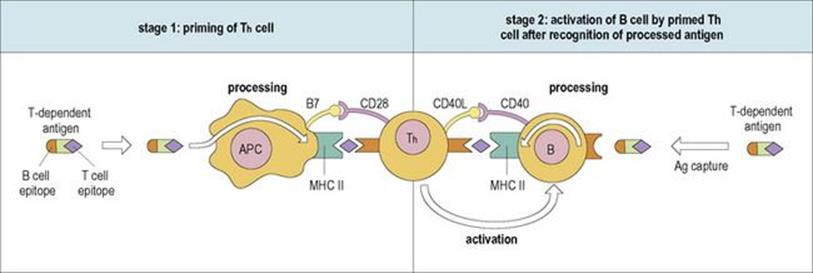
Figure 11.11 The mechanism by which T-helper (Th) cells are primed and then stimulate B cells to synthesize antibody to T-dependent antigens with the help of the cognate co-stimulatory pairs B7/CD28 and CD40L/CD40. See text for a detailed description of the sequence of events. Ag, antigen; APC, antigen-presenting cell; MHC, major histocompatability complex; CD40L, CD40 ligand.
It should be noted that although the Th cell recognizes a processed determinant of the antigen, the B cell is programmed to make only antibody with the same specificity as its surface receptor, and therefore the antibodies that finally result will be directed against the epitope on the antigen recognized by the B-cell surface receptor.
B cells proliferate, are selected for high affinity and differentiate into plasma cell precursors and memory cells in the germinal centres.
Histological features of the important centre for antibody formation, the germinal centre, were presented in Figure 11.3. In essence, the germinal centre consists of a dark zone and a light zone: the dark zone is the site where one or a few B cells enter the primary lymphoid follicle and undergo active proliferation leading to clonal expansion. These B cells are termed ‘centroblasts’ and undergo a process of ‘somatic hypermutation’, which leads to the generation of cells with a wide range of affinities for antigen. In the light zone, B cells (‘centrocytes’) encounter the antigen on the surface of follicular dendritic cells (see Fig. 11.3E) and only those cells with higher affinity for antigen survive. Cells with mutated antibody receptors of lower affinity die by apoptosis and are phagocytosed by germinal centre macrophages.
Selected centrocytes interact with germinal centre CD4+ Th cells and undergo ‘class switching’ (i.e. replacement of their originally expressed immunoglobulin heavy chain constant region genes by another class – for instance IgM to IgG, IgA or IgE – which subserve different functions (cf.Table 10.1).
The selected germinal centre B cells differentiate into ‘memory B cells’ or ‘plasma cell’ precursors and leave the germinal centre (Fig. 11.12).
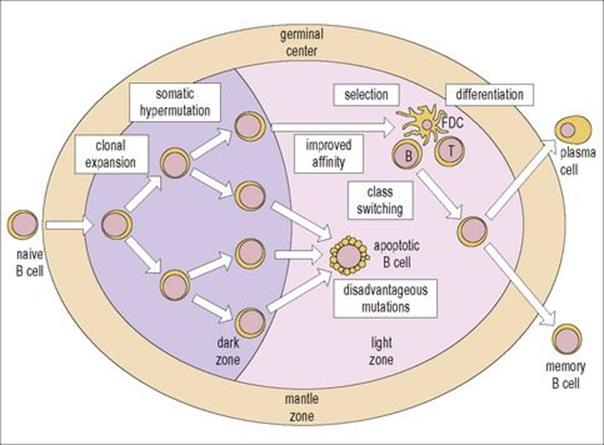
Figure 11.12 Structure and function of the germinal center. One or a few B cells (founder cells) in the dark zone proliferate actively. This proliferation leads to clonal expansion and is accompanied by somatic hypermutation of the immunoglobulin V region genes. B cells with the same specificity, but various affinities, are therefore generated. In the light zone, B cells with disadvantageous mutations or with low affinity undergo apoptosis (Fig. 11.3F) and are phagocytosed by macrophages. Cells with appropriate affinity encounter the antigen on the surface of the follicular dendritic cells (FDCs) and, with the help of CD4+ T cells, undergo class switching, leaving the follicle as memory B cells or plasma cells precursors.
(Reproduced from Male D, Brostoff J, Roth DB, Roitt I. Immunology, 7th edition, 2006. Mosby Elsevier, with permission.)
Cytokines
Cytokines are soluble intercellular communication factors in the immune response
Interactions between the APC, the CD4 Th cell and the B cell are effected by the recognition of processed antigen in association with MHC class II molecules by the TCR and by cognate co-stimulatory surface interactions, B7/CD28 and CD40L/CD40, respectively, as indicated in Figure 11.11. Following this recognition process, the cells become activated and proliferate by releasing soluble factors termed cytokines (Table 11.2), which react with appropriate complementary surface receptors on the target cell. For example, in the activated T cell, the gene encoding the IL-2 receptor (IL-2R) is derepressed and the IL-2R molecule is expressed on the surface of the lymphocytes. A subpopulation of Th cells is also induced to synthesize IL-2, which acts as a growth factor for T cells by combining with the IL-2R, causing proliferation (see Fig. 11.7).
Table 11.2 Known cytokines (hormones of the immune system) and their actions
|
Factor |
Source |
Actions |
|
IL-1 α/β |
Macrophages |
Acute phase proteins |
|
IL-2 |
T cells |
T-cell proliferation |
|
IL-3 |
T cells |
Pluripotent growth |
|
IL-4 |
T cells |
B-cell proliferation and IgE selection, Th1 suppression |
|
IL-5 |
T cells |
B-cell growth, IgA and eosinophil differentiation |
|
IL-6 |
T cells |
B-cell differentiation, induce acute phase proteins |
|
IL-7 |
T cells |
B- and T-cell proliferation |
|
IL-8 |
T cells |
Chemotaxis and activation of PMN |
|
IL-9 |
T cells |
Mast cell growth |
|
IL-10 |
T cells |
Inhibition of Th1 cytokine production |
|
IL-11 |
BM stromal cells |
Osteoclast formation, CSF inhibits proinflammatory cytokine production |
|
IL-12 |
Monocytes, Mφ |
Induction of Th1 cells |
|
IL-13 |
T cells |
Inhibits mononuclear phagocyte inflammation: proliferation and differentiation of B cells |
|
IL-14 |
T cells |
Proliferation of activated B cells, inhibits Ig secretion |
|
IL-15 |
Antigen-presenting cells |
Proliferation of T-, NK and activated B cells; maintenance of T-memory cells |
|
IL-16 |
CD8+ T cells and eosinophils |
Chemotaxis of CD4 T cells |
|
IL-17 |
CD4+ T cells |
Proinflammatory; stimulates production of cytokines including TNFα, IL-1β, IL-6, IL-8, G-CSF |
|
IL-18 |
Macrophages |
Induces IFNγ production by T cells; enhances NK cytotoxicity |
|
IL-21 |
Th cells |
NK differentiation; B activation; T cell co-stimulation induces acute phase reactants |
|
IL-22 |
T cells |
Inhibits IL-4 production by Th 2; induces production of antimicrobial proteins by epithelial cells |
|
IL-23 |
Dendritic cells |
Induces proliferation and IFNγ production by Th1 T cell; induces proliferation of memory cells |
|
IFNα |
Leukocytes |
Antiviral, stimulates IL-12 production and NK cells, induction MHC class I, anti-proliferative |
|
IFNβ |
Fibroblasts, epithelia |
Antiviral, induction MHC class I, anti-proliferative |
|
IFNγ |
T cells, NK cells |
Antiviral, activation of macrophages, inhibition of Th 2 cells, MHC class I and II induction |
|
TNFα |
Monocytes, T cells |
Cytotoxicity, cachexia, fever |
|
Lymphotoxin (TNFβ) |
T cells |
Cytotoxicity, cachexia, fever |
|
TGFβ |
T cells/macrophages |
Inhibits activation of NK and T cells, macrophages; inhibits proliferation of B and T cells, promotes wound healing |
|
GM-CSF |
T cells |
Growth of granulocytes and monocytes |
|
G-CSF |
Macrophages |
Growth of granulocytes |
|
M-CSF |
Macrophages |
Growth of monocytes |
|
MIF |
T cells, macrophages |
Migration inhibition and activation of macrophages |
|
Steel factor |
BM stromal cells |
Stem cell division (c-kit ligand) |
BM, bone marrow; G-CSF, granulocyte colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; IFN, interferon; IL, interleukin; M-CSF, macrophage colony stimulating factor; NK, natural killer cell; PMN, polymorphonuclear lymphocyte; TGF, transforming growth factor; TNFβ, tumour necrosis factor β.
Cytokine production helps to define T-helper subsets (cf. Table 10.2)
Helper T-cell clones generated by antigenic stimulation can be divided into three main types with distinct cytokine secretion phenotypes (Fig. 11.13). This makes biological sense in that Th1 cells producing cytokines such as IFNγ would be especially effective against intracellular infections with viruses and organisms which grow in macrophages, while Th2 cells are very good helpers for B cells and would seem to be adapted for defence against parasites which are vulnerable to IL-4-switched IgE, IL-5-induced eosinophilia and IL-3/4-stimulated mast cell proliferation. The skewing of phenotype towards the extreme Th1/Th2 patterns occurs during the immune response and is partly determined by the nature of the antigen stimulus. There is mutual antagonism between these two subsets in that IL-4 down-regulates Th1 cells and IFNγ suppresses the activity of Th2 lymphocytes. The third helper subset, Th17, secretes powerfully proinflammatory cytokines, and worthy of note is the production of IL-22 which stimulates epithelial cells to produce microbicidal proteins active against bacteria and fungi. It now appears that these cells play a prominent role in the pathogenesis of several autoimmune disorders, but that is another story. Attention has been drawn to the existence of regulatory T-cell subsets which can mediate immunosuppressive effects and have been implicated in the maintenance of self-tolerance (see below). Figure 11.14shows the broad sweep of the cytokine network and the involvement of many different cell types.
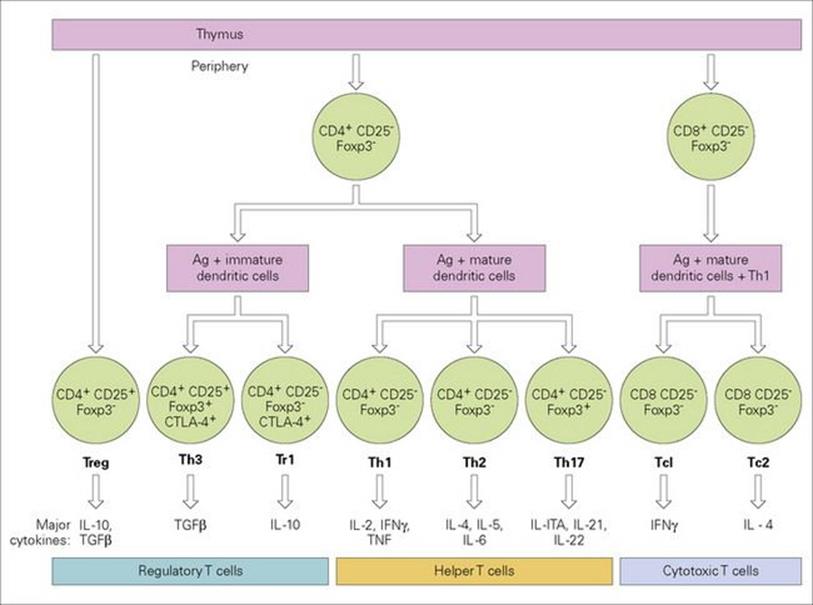
Figure 11.13 Differentiation of thymus-derived T cells into effector subsets and their major secretory cytokine patterns. The various cytokines are listed in Table 11.2. IL-10 is not listed; although classed as a Th2 cytokine in the mouse, it is produced by Th1 and Th2 cells in the human. γδ T cells differentiate as a distinct line from double negative (CD4− CD8−) thymic precursors as do NKT cells. Activation of naive cells to become effectors is always accompanied by generation of memory cells. CD25, IL-2 receptor α-chain; Foxp3, forkhead/winged helix transcription factor, mutations in which lead to dysregulation and autoimmunity; CTLA-4, a down-regulatory factor which binds the B7 co-stimulator (see Fig. 11.9).

Figure 11.14 Cellular interactions mediated by cytokines. As described above, T-helper (Th) cells tend to skew into three major subsets: Th1, producing interleukin-2 (IL-2) and interferon-γ (IFNγ), which activate macrophage-mediated chronic inflammatory reactions; Th2, producing IL-4, IL-5 and IL-6, which act to support B-cell antibody responses and Th17 secreting proinflammatory cytokines including IL-22 which stimulate the production of bactericidal and fungicidal proteins by epithelial cells (space and a desire not to further complicate the diagram have precluded Th17 inclusion in the figure). G-CSF, granulocyte colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; H2O2, hydrogen peroxide; LS, lymphoid stem cell; M-CSF, macrophage colony stimulating factor; MS, myeloid stem cell; NK, natural killer cell; NO, nitric oxide; PC, plasma cell; PMN, polymorphonuclear lymphocyte; SC, stem cell; Tc, cytotoxic T cell; TGFβ, transforming growth factor beta; TNF, tumour necrosis factor.
(Adapted from: Playfair, J. H. L. (2001) Immunology at a glance, Oxford: Blackwell Science.)
The recruitment of the different cells participating in the immune response to the optimal anatomical location is mediated by a very large number of relatively low molecular weight chemoattractant cytokines, termed chemokines, which act through surface receptors on their target cell. Families of chemokines are based on the spacing of conserved cysteine (C) residues. Thus, α-chemokines have a CXC structure and β-chemokines a CC structure. The receptors for the CXC chemokines are designated CXCR1, CXCR2 and so on and of course receptors for CC chemokines are CCR1 etc. Until recently, most chemokines had a descriptive name and acronym such as macrophage chemotactic protein (MCP-1), now designated CCL2, meaning it is a ligand for the CCR receptor family. Other prominent chemokines include IL-8 (now CXCL8), which potently attracts neutrophils, and RANTES (now CCL5), a general attractant for T, NK and dendritic cells plus monocytes, eosinophils and basophils to inflammatory sites, and which binds to CCR5, a co-receptor used in the entry of macrophage-tropic strains of HIV-1 into cells.
Regulatory mechanisms
Unlimited expansion of clones must be checked by regulatory mechanisms
Once lymphocyte clones are activated by antigen, they clearly cannot be allowed to go on dividing indefinitely, otherwise they would completely fill the body of the host. There are therefore several mechanisms regulating the expansion of these dividing lymphocytes.
One of the most important factors controlling the immune response is the concentration of antigen. There is, of course, a distinct evolutionary advantage in a system where the immune response is switched on by antigen and switched off when the antigen is no longer present. It is perhaps not surprising then that selective processes have guided the production of such a system in which the immune response is antigen-driven through the direct effect of antigen on the lymphocyte receptors. As the antigen is eliminated by metabolic catabolism and by clearance through the immune response, the stimulus to the immune system disappears.
Antibody itself has feedback potential
Immunoglobulin M (IgM) produced early in the response has a positive feedback, stimulating the response in its fledgling stages. In contrast, IgG in sufficient concentrations produces negative feedback and acts to down-regulate the immune response partly by antigen removal and, significantly, by cross-linking antigen bound to B-cell surface receptors with the down-regulatory IgG Fc receptor (FcγRIIB) through the VH and Fc domains on the IgG specific antibody molecule respectively (Fig. 11.15).

Figure 11.15 B-cell downregulation. The inhibitory FcγRIIB receptor is activated by the cross-linking through feedback IgG antibody and specific antigen as shown.
Immune responses can be controlled by regulatory T cells
These cells function to prevent T cells, and by implication also B cells, from getting out of hand when responding to antigen and act to prevent autoimmunity by maintaining self-tolerance (see Fig. 11.17). They do not prevent initial T-cell activation but inhibit sustained responses and prevent chronic and potentially damaging immunopathology. Suppression is largely mediated through secretion of IL-10 and/or TGFβ.
A naturally occurring population of regulatory CD4+ cells (Tregs) expressing high levels of CD25 and the transcription factor Foxp3 is generated in the thymus (see Fig. 11.13), and suppresses T-helper cells by direct cell–cell contact. Their role in the maintenance of self-tolerance is revealed by the development of autoimmunity induced by experimental depletion of this subset, or by mutations in the Foxp3 gene. Induced Th3 and Tr1 cells are generated in the periphery by contact with antigen-pulsed immature dendritic cells and suppress T helpers by TGFβ or IL-10 cytokines, respectively.
An overview of the factors regulating immune responses is presented in Figure 11.16.
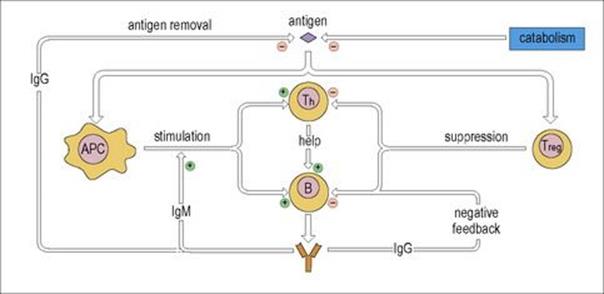
Figure 11.16 Regulation of the immune response. T help for cell-mediated immunity is subject to similar regulation. APC, antigen-presenting cell.
Tolerance mechanisms
Tolerance mechanisms prevent immunologic self-reactivity
To avoid reaction against the body’s own components, it is essential for the immune system to develop non-reactivity or ‘tolerance’ to self molecules. In essence, it is thought that cells that are autoreactive are:
• eliminated by some form of clonal deletion
• made anergic early in the life of the cell
• sometimes silenced through T-regulatory cells later in life (Fig. 11.17).
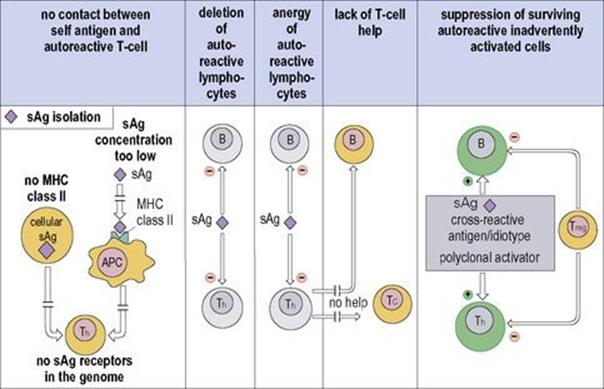
Figure 11.17 Mechanisms of self-tolerance. Self antigens (sAg) will not stimulate autoreactive Th cells if they are anatomically isolated, or if there is too low a concentration of processed peptide-major histocompatibility complex class II (MHC II) molecules, or if there is no MHC II on the cell. Both B and T cells can be silenced by clonal deletion or made anergic (still living, but unresponsive) by contact with self antigen. Too low a concentration of presented sAg will fail to silence differentiating immature lymphocytes bearing the cognate receptors, leading to the survival of populations of autoreactive T and B cells. Th cells are the most readily tolerized population, and surviving autoreactive B cells and cytotoxic T (Tc) cells cannot function without T-cell help. Furthermore, inadvertent stimulation of surviving autoreactive cells may be checked by regulatory T cells (Treg). Cells that are dead, unreactive or suppressed are shown in grey. APC, antigen-presenting cell.
(Modified from: Delves P. J. et al. (2006) Roitt’s Essential Immunology, 11th edn. Oxford: Blackwell Science.)
T cells are more readily tolerized than B cells at a given antigen concentration
There is extremely good evidence that self molecules in the thymus can lead to the deletion or ‘anergy’ of the specific T-cell clone, although autoreactive T cells will survive if the concentration of MHC/self-peptide on the appropriate antigen-presenting cell is too low. B cells in contact with a relatively high concentration of self proteins are also subject to clonal deletion or anergy, but there is less need to tolerize other B cells in the sense that autoreactive B cells directed to thymus-dependent antigens will be unable to respond (helpless) if the corresponding Th cells to that molecule have been tolerized, be it through clonal deletion or suppression by T regulators (Fig. 11.17).
Unresponsiveness will also result if self components cannot be seen or recognized by the immune system. This may occur because over a long period of time the repertoire has lost the genes giving rise to autoreactive receptors. However, even if autoreactive T cells are present, they will not be activated if the self antigen (sAg) is anatomically secluded or is not presented in processed form in combination with MHC class II molecules in adequate concentrations. Therefore, they will also be unable to react with processed sAg presented on the surface of cells that do not express class II. Since most cells express class I molecules, it seems reasonable to assume that the cytotoxic T (Tc) cells capable of reacting against cells expressing processed intracellular components have been deleted, are helpless or are suppressed. As mentioned above, the development of autoimmunity following deletion of the CD4+25+ population and normalization after restoration of this subset strongly suggest that autoreactive cells arising through inadvertent stimulation can be monitored and controlled by these regulatory T cells.
![]()
 Key Facts
Key Facts
• Each lymphocyte expresses either antibody or a TCR with a single specificity for antigen.
• A lymphocyte bearing a complementary antibody or TCR on its surface will bind antigen, be activated, proliferate to form a clone, and differentiate into antibody-forming cells or effectors of cell-mediated immunity, and also form a large pool of memory cells.
• Second contact with antigen stimulates the pool of memory cells to produce a larger and faster response than the primary reaction. Therefore, vaccination with a benign form of the antigen prepares the individual for an effective response on second contact with the antigen during a natural infection.
• Many antigens require T-cell help before they can activate B cells, and subsequent proliferation is mediated by a variety of soluble cytokines.
• Unlimited expansion of clones is restricted by antigen concentration, antibody feedback, regulatory T cells and apoptosis.
• Reactivity to self is prevented by a variety of tolerance mechanisms.
![]()