Medical Microbiology
Section 3 The conflicts
13 Entry, exit and transmission
Introduction
Microorganisms must attach to, or penetrate, the host’s body surfaces
The mammalian host can be considered as a series of body surfaces (Fig. 13.1). To establish themselves on or in the host, microorganisms must either attach to, or penetrate, one of these body surfaces. The outer surface, covered by skin or fur, protects and isolates the body from the outside world, forming a dry, horny, relatively impermeable outer layer. Elsewhere, however, there has to be more intimate contact and exchange with the outside world. Therefore, in the alimentary, respiratory and urogenital tracts, where food is absorbed, gases exchanged and urine and sexual products released, respectively, the lining consists of one or more layers of living cells. In the eye, the skin is replaced by a transparent layer of living cells, the conjunctiva. Well-developed cleansing and defence mechanisms are present at all these body surfaces, and entry of microorganisms always has to occur in the face of these natural mechanisms. Successful microorganisms therefore possess efficient mechanisms for attaching to, and often traversing, these body surfaces.
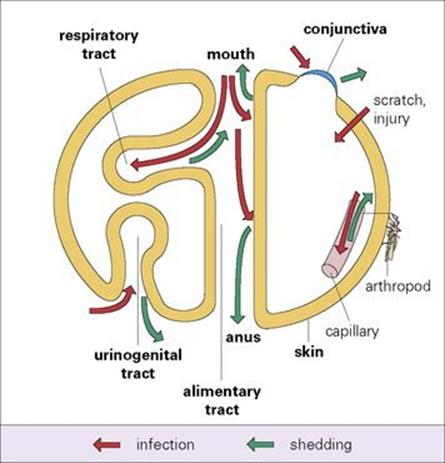
Figure 13.1 Body surfaces as sites of microbial infection and shedding.
Receptor molecules
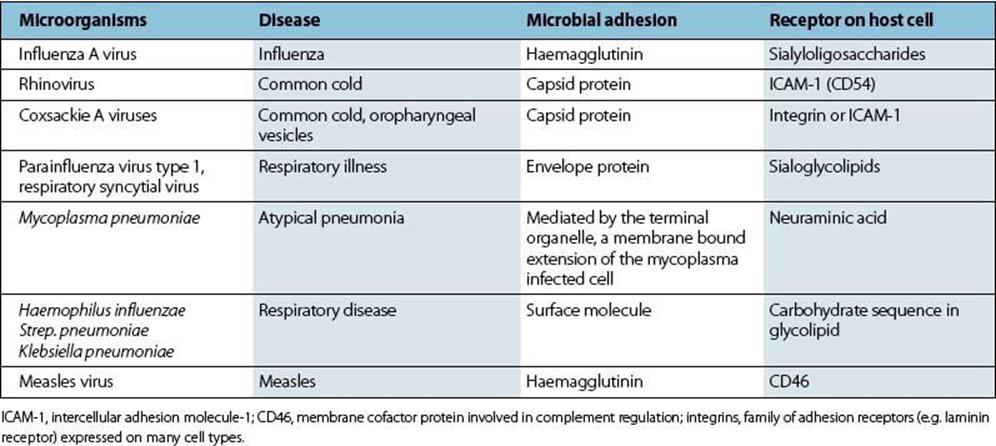
There are often specific molecules on microbes that bind to receptor molecules on host cells, either at the body surface (viruses, bacteria) or in tissues (viruses). These receptor molecules, of which there may be more than one, are not present for the benefit of the virus or other infectious agent; they have specific functions in the life of the cell. Very occasionally, the receptor molecule is present only in certain cells, which are then uniquely susceptible to infection. Examples include the CD4 molecule and the CCR5 beta-chemokine receptor for HIV, the C3d receptor (CR2) for Epstein–Barr virus, and alpha-dystroglycan seems to act as receptor for M. leprae in Schwann cells (the same receptor can be used by arenaviruses). In these cases, the presence of the receptor molecule determines microbial tropism and accounts for the distinctive pattern of infection. Receptors are therefore critical determinants of cell susceptibility, not only at the body surface, but in all tissues. After binding to the susceptible cell, the microorganism can multiply at the surface (mycoplasma, Bordetella pertussis) or enter the cell and infect it (viruses, chlamydia; see Ch. 15).
Exit from the body
Microorganisms must also exit from the body if they are to be transmitted to a fresh host. They are either shed in large numbers in secretions and excretions or are available in the blood for uptake, for example by blood-sucking arthropods or needles.
Sites of entry
Skin
Microorganisms gaining entry via the skin may cause a skin infection or infection elsewhere
Microorganisms which infect or enter the body via the skin are listed in Table 13.1. On the skin, microorganisms other than residents of the normal flora (see Ch. 8) are soon inactivated, especially by fatty acids (skin pH is about 5.5), and probably by substances secreted by sebaceous and other glands, and certain peptides formed locally by keratinocytes protect against invasion by group A streptococci. Materials produced by the normal flora of the skin also protect against infection. Skin bacteria may enter hair follicles or sebaceous glands to cause styes and boils, or teat canals to cause staphylococcal mastitis.
Table 13.1 Microorganisms that infect via the skin
|
Microorganism |
Disease |
Comments |
|
Arthropod-borne viruses |
Various fevers |
150 distinct viruses, transmitted by bite of infected arthropod |
|
Rabies virus |
Rabies |
Bite from infected animals |
|
Human papillomaviruses |
Warts |
Infection restricted to epidermis |
|
Staphylococci |
Boils |
Commonest skin invaders |
|
Rickettsia |
Typhus, spotted fevers |
Infestation with infected arthropod |
|
Leptospira |
Leptospirosis |
Contact with water containing infected animals’ urine |
|
Streptococci |
Impetigo, erysipelas |
Concurrent pharyngeal infection in one-third of cases |
|
Bacillus anthracis |
Cutaneous anthrax |
Systemic disease following local lesion at inoculation site |
|
Treponema pallidum and T. pertenue |
Syphilis, yaws |
Warm, moist skin susceptible |
|
Yersinia pestis, Plasmodia |
Plague, malaria |
Bite from infected rodent flea or mosquito |
|
Trichophyton spp. and other fungi |
Ringworm, athlete’s foot |
Infection restricted to skin, nails, hair |
|
Ancylostoma duodenale (or Necator americanus) |
Hookworm |
Silent entry of larvae through skin of, e.g. foot |
|
Filarial nematodes |
Filariasis |
Bite from infected mosquito, midge, blood-sucking fly |
|
Schistosoma spp. |
Schistosomiasis |
Larvae (cercariae) from infected snail penetrate skin during wading or bathing |
Some remain restricted to the skin (papillomaviruses, ringworm), whereas others enter the body after growth in the skin (syphilis) or after mechanical transfer across the skin (arthropod-borne infections, schistosomiasis).
Several types of fungi (the dermatophytes) infect the non-living keratinous structures (stratum corneum, hair, nails) produced by the skin. Infection is established as long as the parasites’ rate of downward growth into the keratin exceeds the rate of shedding of the keratinous product. When the latter is very slow, as in the case of nails, the infection is more likely to become chronic.
Wounds, abrasions or burns are more common sites of infection. Even a small break in the skin can be a portal of entry if virulent microorganisms such as streptococci, water-borne leptospira or blood-borne hepatitis B virus are present at the site. A few microbes, such as leptospira or the larvae of Ancylostoma and Schistosoma, are able to traverse the unbroken skin by their own activity.
Biting arthropods
Biting arthropods such as mosquitoes, ticks, fleas and sandflies (see Ch. 27) penetrate the skin during feeding and can thus introduce infectious agents or parasites into the body. The arthropod transmits the infection and is an essential part of the life cycle of the microorganism. Sometimes the transmission is mechanical, the microorganism contaminating the mouth parts without multiplying in the arthropod. In most cases, however, the infectious agent multiplies in the arthropod and, as a result of millions of years of adaptation, causes little or no damage to that host. After an incubation period, it appears in the saliva or faeces and is transmitted during a blood feed. The mosquito, for instance, injects saliva directly into host tissues as an anticoagulant, whereas the human body louse defecates as it feeds, and Rickettsia rickettsii, which is present in the faeces, is introduced into the bite wound when the host scratches the affected area.
The conjunctiva
The conjunctiva can be regarded as a specialized area of skin. It is kept clean by the continuous flushing action of tears, aided every few seconds by the windscreen wiper action of the eyelids. Therefore, the microorganisms that infect the normal conjunctiva (chlamydia, gonococci) must have efficient attachment mechanisms (see Ch. 25). Interference with local defences due to decreased lacrimal gland secretion or conjunctival or eyelid damage allows even non-specialist microorganisms to establish themselves. Contaminated fingers, flies, or towels carry infectious material to the conjunctiva, examples including herpes simplex virus infections leading to keratoconjunctivitis or chlamydial infection resulting in trachoma. Antimicrobial substances in tears, including lysozyme, an enzyme, and certain peptides have a defensive role.
Respiratory tract
Some microorganisms can overcome the respiratory tract’s cleansing mechanisms
Air normally contains suspended particles, including smoke, dust and microorganisms. Efficient cleansing mechanisms (see Chs 18 and 19) deal with these constantly inhaled particles. With about 500–1000 microorganisms/m3 inside buildings, and a ventilation rate of 6 l/min at rest, as many as 10 000 microorganisms/day are introduced into the lungs. In the upper or lower respiratory tract, inhaled microorganisms, like other particles, will be trapped in mucus, carried to the back of the throat by ciliary action, and swallowed. Those that invade the normal healthy respiratory tract have developed specific mechanisms to avoid this fate.
Interfering with cleansing mechanisms
The ideal strategy is to attach firmly to the surfaces of cells forming the mucociliary sheet. Specific molecules on the organism (often called adhesins) bind to receptor molecules on the susceptible cell (Fig. 13.2). Examples of such respiratory infections are given in Table 13.2.
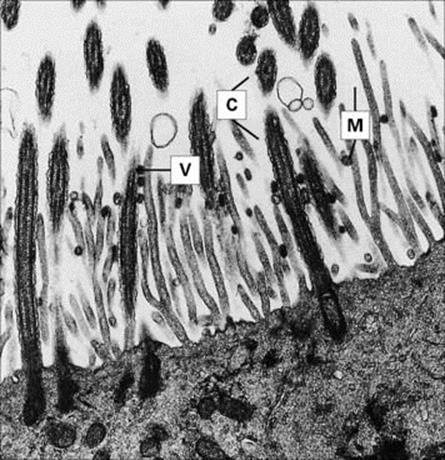
Figure 13.2 Influenza virus attachment to ciliated epithelium. Influenza virus particles (V) attached to cilia (C) and microvilli (M). Electron micrograph of thin section from organ culture of guinea pig trachea 1 h after addition of the virus.
(Courtesy of R.E. Dourmashkin.)
Table 13.2 Microbial attachment in the respiratory tract
Inhibiting ciliary activity is another way of interfering with cleansing mechanisms. This helps invading microorganisms establish themselves in the respiratory tract. B. pertussis, for instance, not only attaches to respiratory epithelial cells, but also interferes with ciliary activity, while other bacteria (Table 13.3) produce various ciliostatic substances of generally unknown nature.
Table 13.3 Interference with ciliary activity in respiratory infections
|
Cause |
Mechanisms |
Importance |
|
Infecting bacteria interfere with ciliary activity (B. pertussis, H. influenzae, P. aeruginosa, M. pneumoniae) |
Production of ciliostatic substances (tracheal cytotoxin from B. pertussis, at least two substances from H. influenzae, at least seven from P. aeruginosa) |
+ + |
|
Viral infection |
Ciliated cell dysfunction or destruction by influenza, measles |
+ + + |
|
Atmospheric pollution (automobiles, cigarette smoking) |
Acutely impaired mucociliary function |
? + |
|
Inhalation of unhumidified air (indwelling tracheal tubes, general anaesthesia) |
Acutely impaired mucociliary function |
+ |
|
Chronic bronchitis, cystic fibrosis |
Chronically impaired mucociliary function |
+ + + |
Although microbes can actively interfere with ciliary activity (first item), a more general impairment of mucociliary function also acts as a predisposing cause of respiratory infection.
Avoiding destruction by alveolar macrophages
Inhaled microorganisms reaching the alveoli encounter alveolar macrophages, which remove foreign particles and keep the air spaces clean. Most microorganisms are destroyed by these macrophages, but one or two pathogens have learnt either to avoid phagocytosis or to avoid destruction after phagocytosis. Tubercle bacilli, for instance, survive in the macrophages, and respiratory tuberculosis is thought to be initiated in this way. Inhalation of as few as 5–10 bacilli is enough. The vital role of macrophages in antimicrobial defences is dealt with more thoroughly in Chapter 14.Alveolar macrophages are damaged following inhalation of toxic asbestos particles and certain dusts, and this leads to increased susceptibility to respiratory tuberculosis.
Gastrointestinal tract
Some microorganisms can survive the intestine’s defences of acid, mucus and enzymes
Apart from the general flow of intestinal contents, there are no particular cleansing mechanisms in the intestinal tract, except insofar as diarrhea and vomiting can be included in this category. Under normal circumstances, multiplication of resident bacteria is counterbalanced by their continuous passage to the exterior with the rest of the intestinal contents. Ingestion of a small number of non-pathogenic bacteria, followed by growth in the lumen of the alimentary canal, produces only relatively small numbers within 12–18 h, the normal intestinal transit time.
Infecting bacteria must attach themselves to the intestinal epithelium (Table 13.4) if they are to establish themselves and multiply in large numbers. They will then avoid being carried straight down the alimentary canal to be excreted with the rest of the intestinal contents. The concentration of microorganisms in faeces depends on the balance between the production and removal of bacteria in the intestine. Vibrio cholerae (Figs 13.3, 13.4) and rotaviruses both establish specific binding to receptors on the surface of intestinal epithelial cells. For V. cholerae, establishment in surface mucus may be sufficient for infection and pathogenicity. The fact that certain microbes infect mainly the large bowel (Shigella spp.) or small intestine (most salmonellae, rotaviruses) indicates the presence of specific receptor molecules on mucosal cells in these sections of the alimentary canal.
Table 13.4 Microbial attachment in the intestinal tract
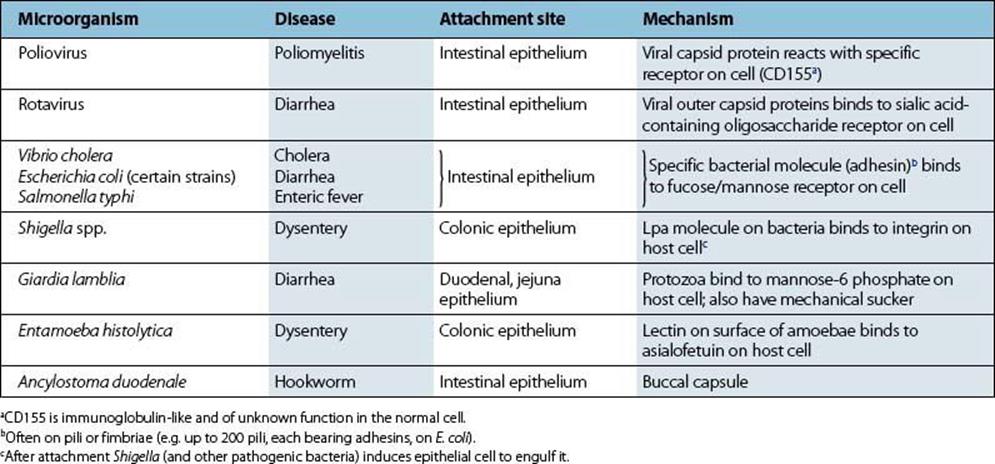
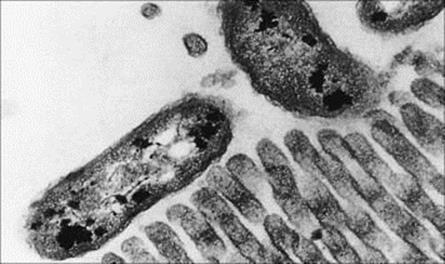
Figure 13.3 Attachment of Vibrio cholerae to brush border of rabbit villus. Thin section electron micrograph (× 10 000).
(Courtesy of E.T. Nelson.)
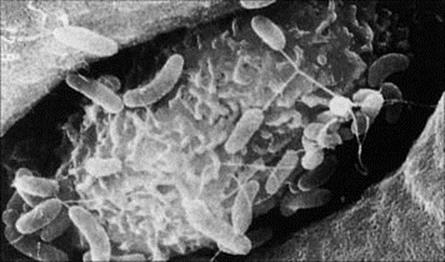
Figure 13.4 Adherence of Vibrio cholerae to M cells in human ileal mucosa.
(Courtesy of T. Yamamoto.)
Infection sometimes involves more than mere adhesion to the luminal surface of intestinal epithelial cells. Shigella flexneri, for example, can only enter these cells from the basal surface. Initial entry occurs after uptake by M cells, and the bacteria then invade local macrophages. This gives rise to an inflammatory response with an influx of polymorphs, which in turn causes some disruption of the epithelial barrier. Bacteria can now enter on a larger scale from the intestinal lumen and invade epithelial cells from below. The bacteria enhance their entry by exploiting the host’s inflammatory response.
Crude mechanical devices for attachment
Crude mechanical devices are used for the attachment and entry of certain parasitic protozoans and worms. Giardia lamblia, for example, has specific molecules for adhesion to the microvilli of epithelial cells, but also has its own microvillar sucking disk. Hookworms attach to the intestinal mucosa by means of a large mouth capsule containing hooked teeth or cutting plates. Other worms (e.g. Ascaris) maintain their position by ‘bracing’ themselves against peristalsis, while tapeworms adhere closely to the mucus covering the intestinal wall, the anterior hooks and sucker playing a relatively minor role for the largest worms. A number of worms actively penetrate into the mucosa as adults (Trichinella, Trichuris) or traverse the gut wall to enter deeper tissues (e.g. the embryos of Trichinella released from the female worm and the larvae of Echinococcus hatched from ingested eggs).
Mechanisms to counteract mucus, acids, enzymes and bile
Successful intestinal microbes must counteract or resist mucus, acids, enzymes and bile
Mucus protects epithelial cells, perhaps acting as a mechanical barrier to infection. It may contain molecules that bind to microbial adhesins, therefore blocking attachment to host cells. It also contains microbe-specific secretory IgA antibodies, which protect the immune individual against infection. Motile microorganisms (V. cholerae, salmonellae and certain strains of E. coli) can propel themselves through the mucus layer and are therefore more likely to reach epithelial cells to make specific attachments; V. cholerae also produces a mucinase, which probably helps its passage through the mucus. Non-motile microorganisms, in contrast, rely on random and passive transport in the mucus layer.
As might be expected, microorganisms that infect by the intestinal route are often capable of surviving in the presence of acid, proteolytic enzymes and bile. This also applies to microorganisms shed from the body by this route (Table 13.5).
Table 13.5 Microbial properties that aid success in the gastrointestinal tract
|
Property |
Examples |
Consequence |
|
Specific attachment to intestinal epithelium |
Poliovirus, rotavirus, Vibrio cholerae |
Microorganism avoids expulsion with other gut contents and can establish infection |
|
Motility |
V. cholerae, certain E. coli strains |
Bacteria travel through mucus and are more likely to reach susceptible cell |
|
Production of mucinase |
V. cholerae |
May assist transit through mucus (neuraminidase) |
|
Acid resistance |
Mycobacterium tuberculosis |
Encourages intestinal tuberculosis (acid labile microorganisms depend on protection in food bolus or in diluting fluid) increased susceptibility in individuals with achlorhydria |
|
Helicobacter pylori |
Establish residence in stomach |
|
|
Enteroviruses (hepatitis A, poliovirus, coxsackieviruses, echoviruses) |
Infection and shedding from gastrointestinal tract |
|
|
Bile resistance |
Salmonella, Shigella, enteroviruses |
Intestinal pathogens |
|
Enterococcus faecalis, E. coli, Proteus, Pseudomonas |
Establish residence |
|
|
Resistance to proteolytic enzymes |
Reoviruses in mice |
Permits oral infection |
|
Anaerobic growth |
Bacteroides fragilis |
Most common resident bacteria in anaerobic environment of colon |
All organisms infecting by the intestinal route must run the gauntlet of acid in the stomach. Helicobacter pylori has evolved a specific defence (Box 13.1). The fact that tubercle bacilli resist acid conditions favours the establishment of intestinal tuberculosis, but most bacteria are acid sensitive and prefer slightly alkaline conditions. For instance, volunteers who drank different doses of V. cholerae contained in 60 mL saline showed a 10 000-fold increase in susceptibility to cholera when 2 g of sodium bicarbonate was given with the bacteria. The minimum disease-producing dose was 108 bacteria without bicarbonate and 104 bacteria with bicarbonate. Similar experiments have been carried out in volunteers with Salmonella typhi, and the minimum infectious dose of 1000–10 000 bacteria was again significantly reduced by the ingestion of sodium bicarbonate. Infective stages of protozoa and worms resist stomach acid because they are protected within cysts or eggs.
![]()
 Box 13.1 Lessons in microbiology
Box 13.1 Lessons in microbiology
How to survive stomach acid: the neutralization strategy of Helicobacter pylori.
This bacterium was discovered in 1983, and was shown to be a human pathogen when two courageous doctors, Warren and Marshall in Perth, Western Australia, drank a potion containing the bacteria and developed gastritis. The infection spreads from person to person by the gastro–oral or fecal–oral route, and 150 years ago, nearly all humans were infected as children. Today, in countries with improved hygiene, this is put off until later in life, until at the age of 50 more than half of the population have been infected. The clinical outcome includes peptic ulcer, gastric cancer and gastric mucosa-associated lymphoid tissue (MALT) lymphoma and host, bacterial and environmental factors are thought to be involved. Genetic susceptibility is implicated in both acquiring and clearing H. pylori (HP) infection. After being eaten, the bacteria have a number of strategies resulting in adaptation to the host gastric mucosa having attached by special adhesins to the stomach wall. These include host mimicry leading to evasion of the host response and genetic variation. Most microbes (e.g. V. cholerae) are soon killed at the low pH encountered in the stomach. H. pylori, however, protects itself by releasing large amounts of urease, which acts on local urea to form a tiny cloud of ammonia round the invader. The attached bacteria induce apoptosis in gastric epithelial cells, as well as inflammation, dyspepsia and occasionally a duodenal or gastric ulcer, so that treatment of these ulcers is by antibiotics rather than merely antacids. Some 90% of duodenal ulcers are due to HP infection, and the rest to aspirin or NSAIDs. The bacteria do not invade tissues, and they stay in the stomach for years, causing asymptomatic chronic gastritis. Up to 3% of infected individuals develop chronic active gastritis and progress to intestinal metaplasia which can lead to stomach cancer. H. pylori was the third bacterium for which the entire genome was sequenced; several gene products have been characterized and key developments include understanding the genetic variation of genes encoding the outer membrane proteins and host adaptation.
![]()
When the infecting microorganism penetrates the intestinal epithelium (Shigella, S. typhi, hepatitis A and other enteroviruses) the final pathogenicity depends upon:
• subsequent multiplication and spread
• toxin production
• cell damage
• inflammatory and immune responses.
Microbial exotoxin, endotoxin and protein absorption
Microbial exotoxins, endotoxins and proteins can be absorbed from the intestine on a small scale. Diarrhea generally promotes the uptake of protein, and absorption of protein also takes place more readily in the infant, which in some species needs to absorb antibodies from milk. As well as large molecules, particles the size of viruses can also be taken up from the intestinal lumen. This occurs in certain sites in particular, such as those where Peyer’s patches occur. Peyer’s patches are isolated collections of lymphoid tissue lying immediately below the intestinal epithelium, which in this region is highly specialized, consisting of so-called M cells (see Fig. 13.4). M cells take up particles and foreign proteins and deliver them to underlying immune cells with which they are intimately associated by cytoplasmic processes.
Urogenital tract
Microorganisms gaining entry via the urogenital tract can spread easily from one part of the tract to another
The urogenital tract is a continuum, so microorganisms can spread easily from one part to another, and the distinction between vaginitis and urethritis, or between urethritis and cystitis, is not always easy or necessary (see Chs 20 and 21).
Vaginal defences
The vagina has no particular cleansing mechanisms, and repeated introductions of a contaminated, sometimes pathogen-bearing foreign object (the penis), makes the vagina particularly vulnerable to infection, forming the basis for sexually transmitted diseases (see Ch. 21). Nature has responded by providing additional defences. During reproductive life, the vaginal epithelium contains glycogen due to the action of circulating estrogens, and certain lactobacilli colonize the vagina, metabolizing the glycogen to produce lactic acid. As a result, the normal vaginal pH is about 5.0, which inhibits colonization by all except the lactobacilli and certain other streptococci and diphtheroids. Normal vaginal secretions contain up to 108/mL of these commensal bacteria. If other microorganisms are to colonize and invade they must either have specific mechanisms for attaching to vaginal or cervical mucosa or take advantage of minute local injuries during coitus (genital warts, syphilis) or impaired defences (presence of tampons, estrogen imbalance). These are the microorganisms responsible for sexually transmitted diseases.
Urethral and bladder defences
The regular flushing action of urine is a major urethral defence, and urine in the bladder is normally sterile.
The bladder is more than an inert receptacle, and in its wall there are intrinsic, but poorly understood, defence mechanisms. These include a protective layer of mucus and the ability to generate inflammatory responses and produce secretory antibodies and immune cells.
Mechanism of urinary tract invasion
The urinary tract is nearly always invaded from the exterior via the urethra, and an invading microorganism must first and foremost avoid being washed out during urination. Specialized attachment mechanisms have therefore been developed by successful invaders (e.g. gonococci, Fig. 13.5). A defined peptide on the bacterial pili binds to a syndecan-like proteoglycan on the urethral cell, and the cell is then induced to engulf the bacterium. This is referred to as parasite-directed endocytosis and also occurs with chlamydia.
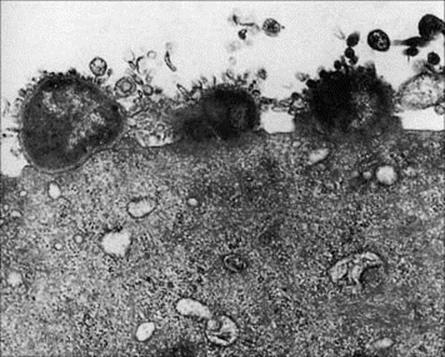
Figure 13.5 Adherence of gonococci to the surface of a human urethral epithelial cell.
(Courtesy of P.J. Watt.)
The foreskin is a handicap in genitourinary infections. This is because sexually transmitted pathogens often remain in the moist area beneath the foreskin after detumescence, giving them increased opportunity to invade. All sexually transmitted infections are more common in uncircumcised males.
Intestinal bacteria (mainly E. coli) are common invaders of the urinary tract, causing cystitis. The genitourinary anatomy is a major determinant of infection (Fig. 13.6). Spread to the bladder is no easy task in the male, where the flaccid urethra is 20 cm long. Therefore, urinary infections are rare in males unless organisms are introduced by catheters or when the flushing activity of urine is impaired (see Ch. 20). The foreskin causes trouble, again, in urinary tract infection by faecal bacteria. These infections are more common in uncircumcised infants because the prepuce may harbour faecal bacteria on its inner surface.
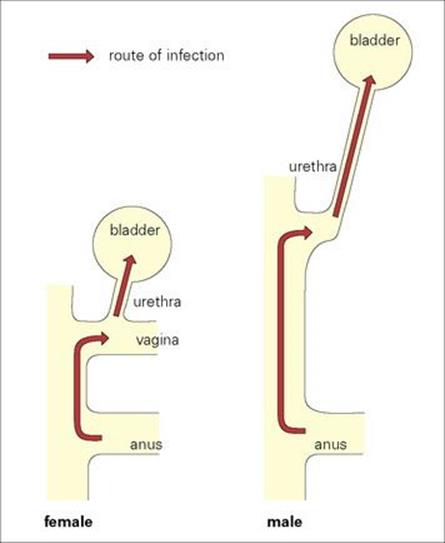
Figure 13.6 The female urinogenital tract is particularly vulnerable to infection with faecal bacteria, mainly because the urethra is shorter and nearer to the anus.
Things are different in females. Not only is the urethra much shorter (5 cm), but it is also very close to the anus (Fig. 13.6), which is a constant source of intestinal bacteria. Urinary infections are about 14 times more common in women, and at least 20% of women have a symptomatic urinary tract infection at some time during their life. The invading bacteria often begin their invasion by colonizing the mucosa around the urethra and probably have special attachment mechanisms to cells in this area. Bacterial invasion is favoured by the mechanical deformation of the urethra and surrounding region that occurs during sexual intercourse, which can lead to urethritis and cystitis. Bacteriuria is about 10 times more common in sexually active women than in nuns.
Oropharynx
Microorganisms can invade the oropharynx when mucosal resistance is reduced
Commensal microorganisms in the oropharynx are described in Chapter 18.
Oropharyngeal defences
The flushing action of saliva provides a natural cleansing mechanism (about 1 L/day is produced, needing 400 swallows), aided by masticatory and other movements of the tongue, cheek and lips. On the other hand, material borne backwards from the nasopharynx is firmly wiped against the pharynx by the tongue during swallowing, and microbes therefore have an opportunity to enter the body at this site. Additional defences include secretory IgA antibodies, antimicrobial substances such as lysozyme, the normal flora, and the antimicrobial activities of leukocytes present on mucosal surfaces and in saliva.
Mechanisms of oropharyngeal invasion
Attaching to mucosal or tooth surfaces is obligatory for both invading and resident microorganisms. For instance, different types of streptococci make specific attachments via lipoteichoic acid molecules on their pili to the buccal epithelium and tongue (resident Streptococcus salivarius), to teeth (resident Strep. mutans), or to pharyngeal epithelium (invading Strep. pyogenes).
Factors that reduce mucosal resistance allow commensal and other bacteria to invade, as in the cases of gum infections caused by vitamin C deficiency, or of Candida invasion (thrush) promoted by changed resident flora after broad-spectrum antibiotics. When salivary flow is decreased for 3–4 h, as between meals, there is a fourfold increase in the number of bacteria in saliva (see Ch. 18). In dehydrated patients, salivary flow is greatly reduced and the mouth soon becomes overgrown with bacteria. As at all body surfaces, there is a shifting boundary between good behaviour by residents and tissue invasion according to changes in host defences.
Exit and transmission
Microorganisms have a variety of mechanisms to ensure exit from the host and transmission
Successful microbes must leave the body and then be transmitted to fresh hosts. Highly pathogenic microbes (e.g. Ebola virus, Legionella pneumophila) will have little impact on host populations if their transmission from person to person is uncommon or ineffective. Nearly all microbes are shed from body surfaces, this being the route of exit to the outside world. Some, however, are extracted from inside the body by vectors, e.g. the blood-sucking arthropods that transmit yellow fever, malaria and filarial worms. Table 13.6 lists the types of infection and their role in the transmission of the microbe and provides a summary of the host defences and the ways in which they are evaded. Transfer from one host to another forms the basis for the epidemiology of infectious disease (see Ch. 31).
Table 13.6 Types of infection and their role in transmission
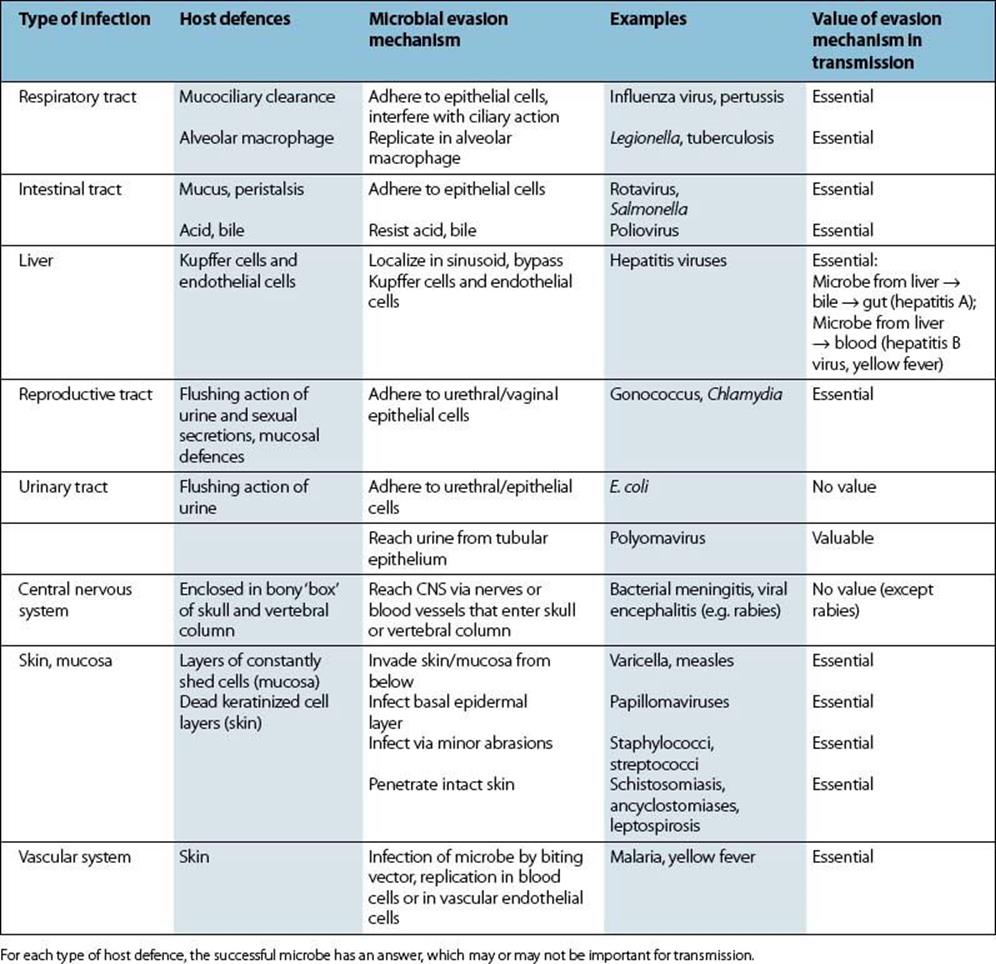
Transmission depends upon three factors:
• the number of microorganisms shed
• the microorganism’s stability in the environment
• the number of microorganisms required to infect a fresh host (the efficiency of the infection).
Number of microorganisms shed
Obviously, the more virus particles, bacteria, protozoa and eggs that are shed, the greater the chance of reaching a fresh host. There are, however, many hazards. Most of the shed microorganisms die, and only an occasional one survives to perpetuate the species.
Stability in the environment
Microorganisms that resist drying spread more rapidly in the environment than those that are sensitive to drying (Table 13.7). Microorganisms also remain infectious for longer periods in the external environment when they are resistant to thermal inactivation. Certain microorganisms have developed special forms (e.g. clostridial spores, amoebic cysts) that enable them to resist drying, heat inactivation and chemical insults, and this testifies to the importance of stability in the environment. If still alive, microorganisms are more thermostable when they have dried. Drying directly from the frozen state (freeze-drying) can make them very resistant to environmental temperatures. The fact that spores and cysts are dehydrated accounts for much of their stability. Microorganisms that are sensitive to drying depend for their spread on close contact, vectors, or contamination of food and water for spread.
Table 13.7 Microbial resistance to drying as a factor in transmission
|
Stability on drying |
Examples |
Consequence |
|
Stable |
Tubercle bacilli |
|
|
Staphylococci |
||
|
Clostridial spores |
|
|
|
Anthrax spores |
||
|
Histoplasma spores |
||
|
Unstable |
Neisseria meningitidis |
|
|
Streptococci |
||
|
Bordetella pertussis |
||
|
Common cold viruses |
||
|
Influenza virus |
||
|
Measles virus |
||
|
Gonococci |
|
|
|
HIV |
||
|
Treponema pallidum |
||
|
Polioviruses |
|
|
|
Hepatitis A |
||
|
Vibrio cholerae |
||
|
Leptospira |
||
|
Yellow fever virus |
|
|
|
Malaria |
||
|
Trypanosomes |
||
|
Larvae/eggs of worms |
Need moist soil (except pinworms) |
Microbes that are already dehydrated such as spores and artificially freeze-dried viruses are also more resistant to thermal inactivation. Spores can survive for years in soil.
Number of microorganisms required to infect a fresh host
The efficiency of the infection varies greatly between microorganisms, and helps explain many aspects of transmission. For instance, volunteers ingesting 10 Shigella dysenteriae bacteria (from other humans) will become infected, whereas as many as 106Salmonella spp. (from animals) are needed to cause food poisoning. The route of infection also matters. A single tissue culture infectious dose of a human rhinovirus instilled into the nasal cavity causes a common cold, and although this dose contains many virus particles, about 200 such doses are needed when applied to the pharynx. As few as 10 gonococci can establish an infection in the urethra, but many thousand times this number are needed to infect the mucosa of the oropharynx or rectum.
Other factors affecting transmission
Genetic factors in microorganisms also influence transmission. Some strains of a given microorganism are therefore more readily transmitted than others, although the exact mechanism is often unclear. Transmission can vary independently of the ability to do damage and cause disease (pathogenicity or virulence).
Activities of the infected host may increase the efficiency of shedding and transmission. Coughing and sneezing are reflex activities that benefit the host by clearing foreign material from the upper and lower respiratory tract, but they also benefit the microorganism. Strains of microorganism that are more able to increase fluid secretions or irritate respiratory epithelium will induce more coughing and sneezing than those less able and will be transmitted more effectively. Similar arguments can be applied to the equivalent intestinal activity: diarrhea. Although diarrhea eliminates the infection more rapidly (prevention of diarrhea often prolongs intestinal infection), from the microbe’s point of view it is a highly effective way of contaminating the environment and spreading to fresh hosts.
Types of transmission between humans
Microorganisms can be transmitted to humans by humans, vertebrates and biting arthropods. Transmission is most effective when it takes place directly from human to human. The most common worldwide infections are spread by the respiratory, faecal–oral or venereal routes. A separate set of infections are acquired from animals, either directly from vertebrates (the zoonoses) or from biting arthropods. Infections acquired from other species are either not transmitted or transmit very poorly from human to human. Types of transmission are illustrated in Figure 13.7.
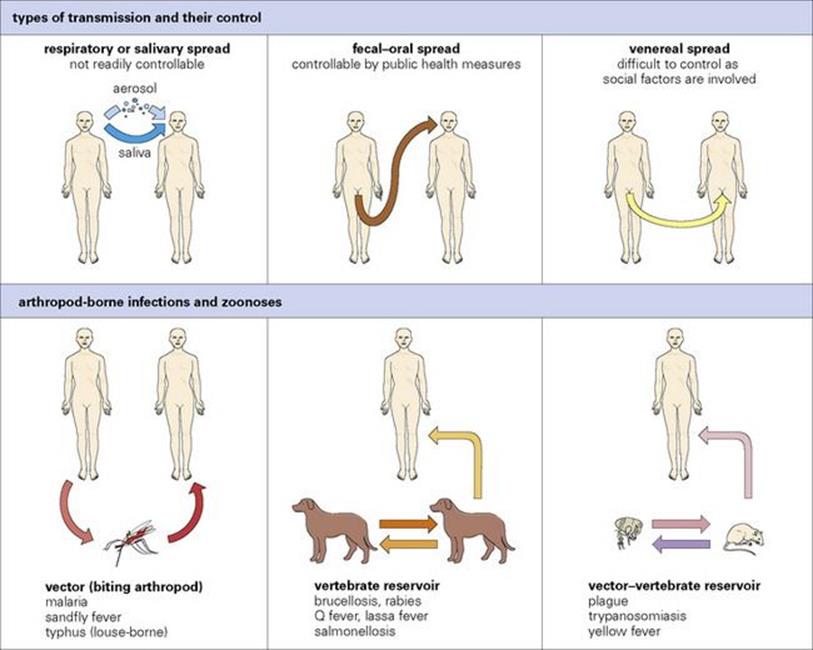
Figure 13.7 Types of transmission and their control. Arthropod-borne infections and zoonoses can be controlled by controlling vectors or by controlling animal infection; there is virtually no person-to-person transmission of these infections (except for pneumonic plague, see Ch. 28).
Transmission from the respiratory tract
Respiratory infections spread rapidly when people are crowded together indoors
An increase in nasal secretions with sneezing and coughing promotes effective shedding from the nasal cavity. In a sneeze (Fig. 13.8) up to 20 000 droplets are produced, and during a common cold, for instance, many of them will contain virus particles.

Figure 13.8 Droplet dispersal following a violent sneeze. Most of the 20 000 particles seen are coming from the mouth.
(Reprinted with permission from: Moulton F. R. (ed.) (1942) Aerobiology. American Association for the Advancement of Science.)
A smaller number of microorganisms (hundreds) are expelled from the mouth, throat, larynx and lungs during coughing (whooping cough, tuberculosis). Talking is a less important source of air-borne particles, but does produce them, especially when the consonants ‘f, p, t and s’ are used. It is surely no accident that many of the most abusive words in the English language begin with these letters, so that a spray of droplets (possibly infectious) is delivered with the abuse!
The size of inhaled droplets determines their initial localization. The largest droplets fall to the ground after travelling approximately 4 m, and the rest settle according to size. Those 10 μm or so in diameter can be trapped on the nasal mucosa. The smallest (1–4 μm diameter) are kept suspended for an indefinite period by normal air movements, and particles of this size are likely to pass the turbinate baffles in the nose and reach the lower respiratory tract.
When people are crowded together indoors, respiratory infections spread rapidly – for example, the common cold in schools and offices and meningococcal infections in military recruits. This is perhaps why respiratory infections are common in winter. The air in ill-ventilated rooms is also more humid, favouring survival of suspended microorganisms such as streptococci and enveloped viruses. Air conditioning is another factor, as the dry air leads to impaired mucociliary activity. Respiratory spread is, in one sense, unique. Material from one person’s respiratory tract can be taken up almost immediately into the respiratory tract of other individuals. This is in striking contrast to the material expelled from the gastrointestinal tract, and helps explain why respiratory infections spread so rapidly when people are indoors.
Handkerchiefs, hands and other objects can carry respiratory infection such as common cold viruses from one individual to another, although coughs and sneezes provide a more dramatic route. Transmission from the infected conjunctiva is referred to in Chapter 25.
The presence of receptors (see Table 13.2) and local temperature as well as initial localization can determine which part of the respiratory tract is infected. For instance, it can be assumed that rhinoviruses arrive in the lower respiratory tract on a large scale, but fail to grow there because, like leprosy bacilli, they prefer the cooler temperature of the nasal mucosa.
Transmission from the gastrointestinal tract
Intestinal infection spreads easily if public health and hygiene are poor
The spread of an intestinal infection is assured if public health and hygiene are poor, the microbe appears in the faeces in sufficient numbers and there are susceptible individuals in the vicinity. Diarrhea gives it an additional advantage, and the key role of diarrhea in transmission has been referred to above. During most of human history, there has been a large-scale recycling of faecal material back into the mouth, and this continues in resource-poor countries. The attractiveness of the faecal–oral route for microorganisms and parasites is reflected in the great variety that are transmitted in this way.
Intestinal infections have been to some extent controlled in resource-rich countries. The great public health reforms of the nineteenth century led to the introduction of adequate sewage disposal and a supply of purified water. For instance, in England 200 years ago, there were no flushing toilets and no sewage disposal and much of the drinking water was contaminated. Cholera and typhoid spread easily, and in London, the Thames became an open sewer. Today, as in other cities, a complex underground disposal system separates sewage from drinking water. Intestinal infections are still transmitted in resource-rich countries, but via food and fingers rather than by water and flies. Therefore, although each year in the UK there are dozens of cases of typhoid acquired on visits to resource-poor countries, the infection is not transmitted to others.
The microorganisms that appear in faeces usually multiply in the lumen or wall of the intestinal tract, but there are a few that are shed into bile. For instance, hepatitis A enters bile after replicating in liver cells.
Transmission from the urogenital tract
Urogenital tract infections are often sexually transmitted
Urinary tract infections are common, but most are not spread via urine. Urine can contaminate food, drink and living space. Examples of some infections that are spread by urine are listed in Table 13.8.
Table 13.8 Human infections transmitted via urine
|
Infection |
Details |
Value in transmission |
|
Schistosomiasis |
Parasite eggs excreted in bladder |
+ + + |
|
Typhoid |
Bacterial persistence in bladder scarred by schistosomiasis |
+ |
|
Polyomavirus infection |
Commonly excreted in urine |
? |
|
Cytomegalovirus infection |
Commonly excreted in infected children |
? |
|
Leptospirosis |
Infected rats and dogs excrete bacteria in urine |
+ + |
|
Lassa fever (and South American haemorrhagic fevers) |
Persistently infected rodent excretes virus in urine |
+ + + |
Schistosomiasis is the major infection transmitted in this way, the eggs undergoing development in snails before reinfecting humans. Viruses are shed in the urine after infecting tubular epithelial cells in the kidney.
Sexually transmitted infections (STIs)
Microorganisms shed from the urogenital tract are often transmitted as a result of mucosal contact with susceptible individuals, typically as a result of sexual activity. If there is a discharge, organisms are carried over the epithelial surfaces and transmission is more likely. Some of the most successful sexually transmitted microorganisms (gonococci, chlamydia) therefore induce a discharge. Other microorganisms are transmitted effectively from mucosal sores (ulcers), e.g. Treponema pallidum and herpes simplex virus. The human papillomaviruses are transmitted from genital warts or from foci of infection in the cervix where the epithelium, although apparently normal, is dysplastic and contains infected cells (see Ch. 21).
The transmission of STIs is determined by social and sexual activity. Changes in the size of the human population and way of life have had a dramatic effect on the epidemiology of STIs. More opportunities to have sexual encounters have arisen due to increasing population density, increased movement of people, the decline of the idea that sexual activity is sinful and the knowledge that STIs are treatable and pregnancy is avoidable. In addition, the contraceptive pill has favoured the spread of STIs by discouraging the use of mechanical barriers to conception. Condoms have been shown to reliably retain herpes simplex virus, HIV, chlamydia and gonococci in simulated coital tests of the syringe and plunger type (see Ch. 21).
STIs are, however, transmitted with far less speed and efficiency than respiratory or intestinal infections. Influenza can be transmitted to a multitude of others during 1 h in a crowded room, or a rotavirus to a score of children during a morning at kindergarten, but STIs can only spread to each person by a separate sexual act. Promiscuity is therefore essential. Frequent sexual activity is not enough without promiscuity because those in a stable partnership can do no more than infect each other. The increased general level of promiscuity in society, together with the huge numbers of sexual partners of certain individuals, such as prostitutes, has led to a dramatic rise in the incidence of STIs.
As almost all mucosal surfaces of the body can be involved in sexual activity, microorganisms have had increasing opportunity to infect new body sites. The meningococcus, a nasopharyngeal resident, has therefore sometimes been recovered from the cervix, the male urethra, and the anal canal, while occasionally gonococci and chlamydia infect the throat and anal canal. The possibilities are illustrated in all their complexity in Figure 13.9, apparently limited only by anatomic considerations. It is no surprise that genito–oro–anal contacts have sometimes allowed intestinal infections such as salmonella, giardia, hepatitis A virus, shigella, and pathogenic amoebae to spread directly between individuals despite good sanitation and sewage disposal.
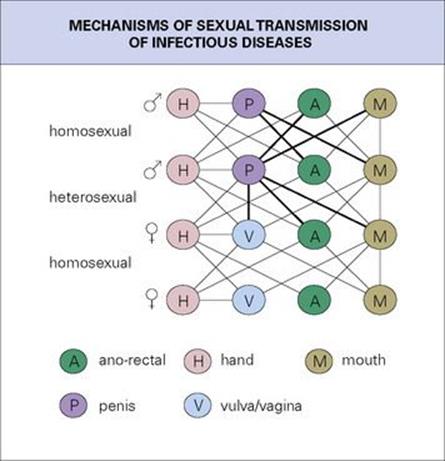
Figure 13.9 The mechanisms of sexual transmission of infection.
(Redrawn from: Wilcox R. R. (1981) The rectum as viewed by the venereologist. Br J Ven Dis 1981; 57:1–6.)
Semen as a source of infection
It might be expected that semen is involved in the transmission of infection, and this is the case in viral infections of animals such as blue tongue and foot and mouth disease. In humans, cytomegalovirus that is shed from the oropharynx is also often present in large quantities in semen, and the fact that it is also recoverable from the cervix suggests that it is sexually transmitted. Hepatitis B virus and HIV are also present in semen.
Perinatal transmission
The female genital tract can also be a source of infection for the newborn child (see Ch. 23). During passage down an infected birth canal, microorganisms can be wiped onto the conjunctiva of the infant or inhaled, leading to a variety of conditions such as conjunctivitis, pneumonia and bacterial meningitis.
Transmission from the oropharynx
Oropharyngeal infections are often spread in saliva
Saliva is often the vehicle of transmission. Microorganisms such as streptococci and tubercle bacilli reach saliva during upper and lower respiratory tract infections, while certain viruses infect the salivary glands and are transmitted in this way. Paramyxovirus, herpes simplex virus, cytomegalovirus and human herpesvirus type 6 are shed into saliva. In young children, fingers and other objects are regularly contaminated by saliva, and each of these infections is acquired by this route. Epstein–Barr virus is also shed into saliva, but is transmitted less effectively, perhaps because it is present only in cells or in small amounts. In resource-rich countries, people often escape infection during childhood, and become infected as adolescents or adults during the extensive salivary exchanges (mean 4.2 mL/h) that accompany deep kissing (see Ch. 18). Saliva from animals is the source of a few infections, and these are included in Table 13.9.
Table 13.9 Human infections transmitted via saliva
|
Microorganism |
Comments |
|
Herpes simplex, paramyxovirus |
Infection generally during childhood |
|
Cytomegalovirus, Epstein–Barr virus |
Adolescent/adult infection is common |
|
Rabies virus |
Shed in saliva of infected dogs, wolves, jackals, vampire bats |
|
Pasteurella multocida |
Bacteria in upper respiratory tract of dogs, cats appear in saliva and are transmitted via bites, scratches |
|
Streptobacillus moniliformis |
Present in rat saliva and infects humans (rat bite fever) |
Transmission from the skin
Skin can spread infection by shedding or direct contact
Dermatophytes (fungi such as those that cause ringworm) are shed from skin and also from hair and nails, the exact source depending on the type of fungus (see Ch. 26). Skin is also an important source of certain other bacteria and viruses, as outlined in Table 13.10.
Table 13.10 Human infections transmitted from the skin
|
Microorganism |
Disease |
Comments |
|
Staphylococci |
Boils, carbuncles, neonatal skin sepsis |
Pathogenicity varies, skin lesions or nose picking are common sources of infection |
|
Treponema pallidum |
Syphilis |
Mucosal surfaces more infectious than skin |
|
Treponema pertenue |
Yaws |
Regular transmission from skin lesions |
|
Streptococcus pyogenes |
Impetigo |
Vesicular (epidermal) lesions crusting over, common in children in hot, humid climates |
|
Staphylococcus aureus |
Impetigo |
Less common; bullous lesions, especially in newborn |
|
Dermatophytes |
Skin ringworm |
Different species infect skin, hair, nails |
|
Herpes simplex virus |
Herpes simplex, cold sore |
Up to 106 infectious units/ml of vesicle fluid |
|
Varicella-zoster virus |
Varicella, zoster |
Vesicular skin lesions occur but transmission is usually respiratorya |
|
Coxsackievirus A16 |
Hand, foot and mouth disease |
Vesicular skin lesions but transmission faecal and respiratory |
|
Papillomaviruses |
Warts |
Many typesb |
|
Leishmania tropica |
Cutaneous leishmaniasis |
Skin sores are infectious |
|
Sarcoptes scabei |
Scabies |
Eggs from burrow transmitted by hand (also sexually) |
a Except in zoster, where a localized skin eruption occurs and the respiratory tract is generally unaffected.
b Generally direct contact, but plantar warts are commonly spread following contamination of floors.
Shedding to the environment
The normal individual sheds desquamated skin scales into the environment at a rate of about 5×108/day, the rate depending upon physical activities such as exercise, dressing and undressing. The fine white dust that collects on indoor surfaces, especially in hospital wards, consists largely of skin scales. Staphylococci are present, and different individuals show great variation in staphylococcal shedding, but the reasons are unknown.
Transmission by direct contact or by contaminated fingers is much more common than following release into the environment, and microorganisms transmitted in this way include potentially pathogenic staphylococci and human papillomaviruses.
Transmission in milk
Milk is produced by a skin gland. Microorganisms are rarely shed into human milk, and examples include HIV, cytomegalovirus and human T-cell lymphotropic virus 1 (HTLV-1), but milk from cows, goats and sheep can be important sources of infection (Table 13.11). Bacteria can be introduced into milk after collection.
Table 13.11 Human infections transmitted via milk
|
Microorganism |
Type of milk |
Importance in transmission |
|
Cytomegalovirus |
Human |
− |
|
HIV |
Human |
+ |
|
HTLV-1 |
Human |
+ |
|
Brucella |
Cow, goat, sheep |
+ + |
|
Mycobacterium bovis |
Cow |
+ + |
|
Coxiella burnetii (Q fever) |
Cow |
+ |
|
Campylobacter jejuni |
Cow |
+ + |
|
Salmonella spp. |
|
+ |
|
Listeria monocytogenes |
||
|
Staphylococcus spp. |
||
|
Streptococcus pyogenes |
||
|
Yersinia enterocolitica |
Human milk is rarely a significant source of infection. All microbes listed are destroyed by pasteurization.
Transmission from blood
Blood can spread infection via arthropods or needles
Blood is often the vehicle of transmission. Microorganisms and parasites spread by blood-sucking arthropods (see below) are effectively shed into the blood. Infectious agents present in blood (hepatitis B and C viruses, HIV) are also transmissible by needles, either in transfused blood or when contaminated needles are used for injections or intravenous drug misuse. Intravenous drug misuse is a well-known factor in the spread of these infections. In addition, at least 12 000 million injections are given each year, worldwide, about 1 in 10 of them for vaccines. Unfortunately, in parts of the resource-poor world, disposable syringes tend to be used more than once, without being properly sterilized in between (‘If it still works, use it again’). To prevent this, the World Health Organization (WHO) encouraged the use of syringes in which, for instance, the plunger cannot be withdrawn once it has been pushed in.
Blood is also the source of infection in transplacental transmission and this generally involves initial infection of the placenta (see Ch. 23).
Vertical and horizontal transmission
Vertical transmission takes place between parents and their offspring
When transmission occurs directly from parents to offspring via, e.g. sperm, ovum, placenta (Table 13.12), milk or blood, it is referred to as vertical. This is because it can be represented as a vertical flow down a page (Fig. 13.10), just like a family pedigree. Other infections, in contrast, are said to be horizontally transmitted, with an individual infecting other individuals by contact, respiratory or faecal–oral spread. Vertically transmitted infections can be subdivided as shown in Table 13.13. Strictly speaking, these infections are able to maintain themselves in the species without spreading horizontally, as long as they do not affect the viability of the host. Various retroviruses are known to maintain themselves vertically in animals (e.g. mammary tumour virus in milk, sperm and ovum of mice), but this does not appear to be important in humans, except possibly for HTLV-1, where milk transfer is important. There are, however, many retrovirus sequences present in the normal human genome known as endogenous retroviruses. These DNA sequences are too incomplete to produce infectious virus particles, but can be regarded as amazingly successful parasites. In addition, some of them may confer benefit, for example, by coding for proteins that help coordinate early stages of fetal development. They presumably do no harm and survive within the human species, watched over, conserved and replicated as part of our genetic constitution.
Table 13.12 Human infections transmitted via the placenta
|
Transplacental transmission of infection |
|
|
Microorganism |
Effect |
|
Rubella virus, cytomegalovirus |
Placental lesion, abortion, stillbirth, malformation |
|
HIV |
Childhood HIV and AIDS |
|
Hepatitis B virus |
Antigen carriage in infant, but most of these infections are perinatal or postnatal |
|
Treponema pallidum |
Stillbirth, congenital syphilis with malformation |
|
Listeria monocytogenes |
Meningoencephalitis |
|
Toxoplasma gondii |
Stillbirth, CNS disease |
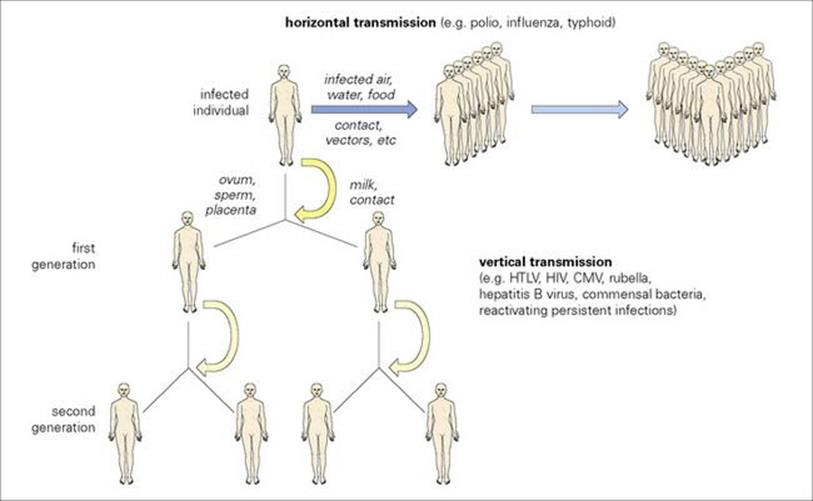
Figure 13.10 Vertical and horizontal transmission by infection. Most infections are transmitted horizontally, as might be expected in crowded human populations. Vertical transmission becomes more important in small isolated communities (see Ch. 17). CMV, cytomegalovirus; HTLV, human T-cell lymphotropic virus.
Table 13.13 Types of vertical transmission
|
Type |
Route |
Examples |
|
Prenatal |
Placenta |
Rubella; cytomegalovirus; syphilis; toxoplasmosis |
|
Perinatal |
Infected birth canal |
Gonococcal/chlamydial conjunctivitis; hepatitis B |
|
Postnatal |
Milk or direct contact |
Cytomegalovirus; hepatitis B virus; HIV, HTLV-1 |
|
Germline |
Viral DNA sequences in human genome |
Many ancient retroviruses |
HTLV, human T-cell lymphotropic virus.
Transmission from animals
Humans and animals share a common susceptibility to certain pathogens
Humans live in daily contact, directly or indirectly, with a wide variety of other animal species, both vertebrate and invertebrate, not only sharing a common environment, but also a common susceptibility to certain pathogens. The degree to which animal contacts transmit infection depends upon the type of environment (urban/rural, tropical/temperate, hygienic/insanitary) and on the nature of the contact. Close contact is made with vertebrate animals used for food or as pets, and with invertebrate animals adapted to live or feed on the human body. Less intimate contact is made with many other species, which nevertheless may transmit pathogens equally well. For convenience, animal-transmitted infections can be divided into two categories:
• those involving arthropod and other invertebrate vectors
• those transmitted directly from vertebrates (zoonoses).
More detailed accounts of these infections are given in Chapters 27 and 28.
Invertebrate vectors
Insects, ticks and mites – the bloodsuckers – are the most important vectors spreading infection
By far the most important vectors of disease belong to these three groups of arthropods. Many species are capable of transmitting infection, and a wide range of organisms is transmitted (Table 13.14). In the past, insects have been responsible for some of the most devastating epidemic diseases, for example, fleas and plague and lice and typhus. Even today, one of the world’s most important infectious diseases, malaria, is transmitted by the Anopheles mosquito. The distribution and epidemiology of these infections are determined by the climatic conditions that allow the vectors to breed and the organism to complete its development in their bodies. Some diseases are therefore purely tropical and subtropical, for example, malaria, sleeping sickness and yellow fever, while others are much more widespread, e.g. plague and typhus.
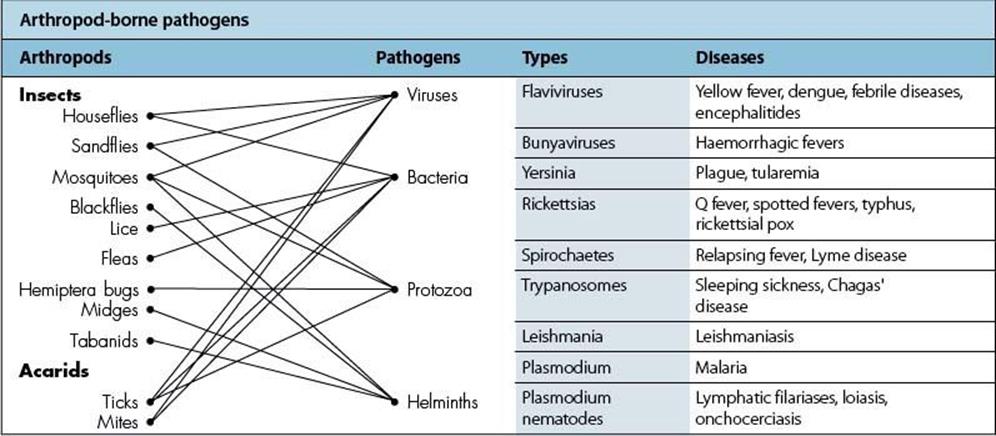
Table 13.14 Arthropod-borne pathogens. Mosquitoes are a major source of infection. (Note that, with the exception of pneumonic plague, none is transmitted from human to human.)
Passive carriage
Insects may carry pathogens passively on their mouth parts, on their bodies, or within their intestines. Transfer onto food or onto the host occurs directly as a result of the insect feeding, regurgitating or defecating. Many important diseases, such as trachoma, can be transmitted in this way by common species such as houseflies and cockroaches.
Blood-feeding species have mouth parts adapted for penetrating skin in order to reach blood vessels or to create small pools of blood (Fig. 13.11). The ability to feed in this way provides access to organisms in the skin or blood. The mouth parts can act as a contaminated hypodermic needle, carrying infection between individuals.
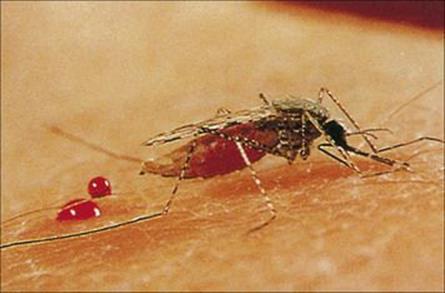
Figure 13.11 Female Anopheles mosquito feeding.
(Courtesy of C.J. Webb.)
Biologic transmission
This is much more common, the blood-sucking vector acting as a necessary host for the multiplication and development of the pathogen. Almost all of the important infections (listed in Table 13.14) are transmitted in this way. The pathogen is reintroduced into the human host, after a period of time, at the next blood meal. Transmission can be by direct injection, usually in the vector’s saliva (malaria, yellow fever), or by contamination from faeces or regurgitated blood deposited at the time of feeding (typhus, plague).
Other invertebrate vectors spread infection either passively or by acting as an intermediate host
Many invertebrates used for food convey pathogens (Fig. 13.12). Perhaps the most familiar are the shellfish (molluscs and crustacea) associated with food poisoning and acute gastroenteritis. These filter feeders accumulate viruses and bacteria in their bodies, taking them in from contaminated waste, and transferring them passively. In other cases, the relationship between the pathogen and the invertebrate is much closer. Many parasites, especially worms, must undergo part of their development in the invertebrate before being able to infect a human. Humans are infected when they eat the invertebrate (intermediate) host. Dietary habits are therefore important in infection.
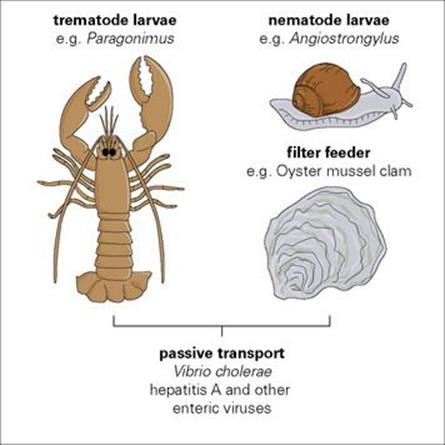
Figure 13.12 Microorganisms transmitted via invertebrates used for food. Filter-feeding molluscs living in estuaries near sewage outlets are a common source of infection.
Aquatic molluscs (snails) are necessary intermediate hosts for schistosomes – the blood flukes. They become infected by larval stages, which hatch from eggs passed into water in the urine or faeces of infected people. After a period of development and multiplication, large numbers of infective stages (cercariae) escape from the snails. These can rapidly penetrate through human skin, initiating the infection that will result in adult flukes occupying visceral blood vessels (see Ch. 30).
Transmission from vertebrates
Many pathogens are transmitted directly to humans from vertebrate animals
Strictly, the term zoonoses can apply to any infection transmitted to humans from infected animals, whether this is direct (by contact or eating) or indirect (via an invertebrate vector). Here, however, zoonoses are used to describe infections of vertebrate animals that can be transmitted directly. Many pathogens are transmitted in this way (Table 13.15) by a variety of different routes including contact, inhalation, bites, scratches, contamination of food or water and ingestion as food.
Table 13.15 Zoonoses: human infections transmitted directly from vertebrates (birds and mammals)
|
Pathogens |
Vertebrate vector |
Diseases |
|
Viruses |
||
|
Arenaviruses |
Mammals |
Lassa fever, lymphocytic choriomeningitis, Bolivian haemorrhagic fever |
|
Poxviruses |
Mammals |
Cowpox, orf |
|
Rhabdoviruses |
Mammals |
Rabies |
|
Bacteria |
||
|
Bacillus anthracis |
Mammals |
Anthrax |
|
Brucella |
Mammals |
Brucella |
|
Chlamydia |
Birds |
Psittacosis |
|
Leptospira |
Mammals |
Leptospirosis (Weil’s disease) |
|
Listeria |
Mammals |
Listeriosis |
|
Salmonella |
Birds, mammals |
Salmonellosis |
|
Mycobacterium tuberculosis |
Mammals |
Tuberculosis |
|
Fungi |
||
|
Cryptococcus |
Birds |
Meningitis |
|
Dermatophytes |
Mammals |
Ringworm |
|
Protozoa |
||
|
Cryptosporidium |
Mammals |
Cryptosporidiosis |
|
Giardia |
Mammals |
Giardiasis |
|
Toxoplasma |
Mammals |
Toxoplasmosis |
|
Helminths |
||
|
Ancylostoma |
Mammals |
Hookworm disease |
|
Echinococcus |
Mammals |
Hydatid disease |
|
Taenia |
Mammals |
Tapeworms |
|
Toxocara |
Mammals |
Toxocariasis (visceral larval migrans) |
|
Trichinella |
Mammals |
Trichinellosis |
A few virus infections acquired from animals (SARS, influenza A H5N1 virus) show poor transmission from person to person, but they may at any time change and develop the capacity for efficient transmission.
The epidemiology of zoonoses depends upon the frequency and the nature of contact between the vertebrate and the human hosts. Some are localized geographically, being dependent, for example, on local food preferences. Where these involve eating uncooked animal products such as fish or amphibia, a variety of parasites (especially tapeworms and nematodes) can be acquired. Others are associated with occupation; for example, if this involves contact with raw animal products (butchers in the case of toxoplasmosis and Q fever) or frequent contact with domestic stock (farm workers in the case of brucellosis and dermatophyte fungi). In urban areas, zoonoses are most likely to be acquired by eating or drinking infected animal products or by contact with dogs, cats and other domestic pets.
Domestic pets or pests?
Dogs and cats are the most common domestic pets, and both are reservoirs of infection for their owners (Fig. 13.13). The pathogens concerned are spread by contact, bites and scratches, by vectors, and by contamination with faecal material. Major infections transmitted in these ways include:
• toxocariasis from dogs
• toxoplasmosis from cats.
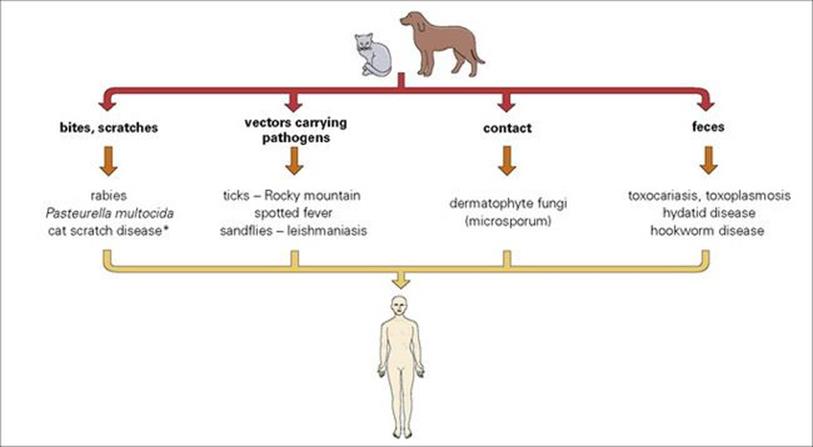
Figure 13.13 Man’s best friends? Zoonoses transmitted from dogs and cats. (*A benign infection, with skin lesions and lymphadenopathy, shown to be due to a bacterium, Bartonella henselae)
Both are almost universal in their distribution.
Humans may acquire hydatid disease from tapeworm eggs passed in dog faeces where dogs are used for herding domestic animals and have access to infected carcasses. In rural areas of many countries this has been, or remains, an important infection.
Many species of birds are kept as pets and some can pass on serious infections to those in contact with them. Contact is usually through inhalation of infected particulate material. Perhaps the most important of these is psittacosis caused by Chlamydophila (formerly Chlamydia) psittaci, which despite the common name ‘parrot fever’ can be acquired from many avian species.
The recent trend in resource-rich countries towards keeping unusual or exotic pets (especially reptiles, exotic birds and mammals) raises new risks of zoonotic infection. Many reptiles, for example, pass human-infective Salmonella spp. in their droppings. Exotic birds and mammals can carry a range of viruses that could be transmitted under the correct conditions. Diagnosis of infections under these circumstances can be difficult if the physician does not know of the existence of such pets.
![]()
 Key Facts
Key Facts
• To establish infection in the host, microbes must attach to, or pass across, body surfaces.
• Many microbes have developed chemical or mechanical mechanisms to attach themselves to the surface of the respiratory, urogenital or alimentary tracts. In the skin, they generally depend upon entry via small wounds or arthropod bites.
• Microbes must exit from the body after replication in order to be transmitted to fresh hosts. This also takes place across body surfaces.
• Efficient shedding of microbes from the skin or respiratory, urogenital or alimentary tracts, or delivery into the blood or dermal tissues for uptake during arthropod feeding, are vital stages in their life cycles.
• Many human infections come from animals, either directly (zoonoses) or indirectly (via blood-sucking arthropods), and the incidence of these infections depends upon exposure to infected animals or arthropods.
![]()