Medical Microbiology
Section 4 Clinical manifestation and diagnosis of infections by body system
26 Infections of the skin, soft tissue, muscle and associated systems
Introduction
Healthy intact skin protects underlying tissues and provides excellent defence against invading microbes
The microbial load of normal skin is kept in check by various factors, as shown in Box 26.1. Alterations in these factors (e.g. prolonged exposure to moisture) upset the ecologic balance of the commensal flora, and predispose to infection.
![]()
Box 26.1  Factors Controlling the Skin’s Microbial Load
Factors Controlling the Skin’s Microbial Load
• the limited amount of moisture present
• acid pH of normal skin
• surface temperature < optimum for many pathogens
• salty sweat
• excreted chemicals such as sebum, fatty acids and urea
• competition between different species of the normal flora.
The number of bacteria on the skin vary from a few hundred/cm2 on the arid surfaces of the forearm and back, to tens of thousands/cm2 on the moist areas, such as the axilla and groin. This normal flora plays an important role in preventing ‘foreign’ organisms from colonizing the skin, but it too needs to be kept in check.
![]()
A small number of microbes cause diseases of muscle, joints or the haemopoietic system. Invasion of these sites is generally from the blood, but the reason for localization to particular tissues is often obscure. Circulating microbes tend to localize in growing or damaged bones (acute osteomyelitis) and in damaged joints, but we do not know why coxsackieviruses or Trichinella spiralis invade muscle. On the other hand, some viruses infect a given target cell, and plasmodia invade erythrocytes because they have specific attachment sites for these cells.
Infections of the skin
In addition to being a structural barrier, the skin is colonized by an array of organisms which forms its normal flora. The relatively arid areas of the forearm and back are colonized with fewer organisms, predominantly Gram-positive bacteria and yeasts. In the moister areas, such as the groin and the armpit, the organisms are more numerous and more varied and include Gram-negative bacteria. The normal flora of the skin plays an important role, as does the normal flora in other body sites, in defending the surface from ‘foreign invaders’.
An appreciation of the structure of the skin helps in understanding the different sorts of infection to which the skin and its underlying tissues are prone (Fig. 26.1). If organisms breach the stratum corneum the host defences are mobilized, the epidermal Langerhans cells elaborate cytokines, neutrophils are attracted to the site of invasion, and complement is activated via the alternative pathway.
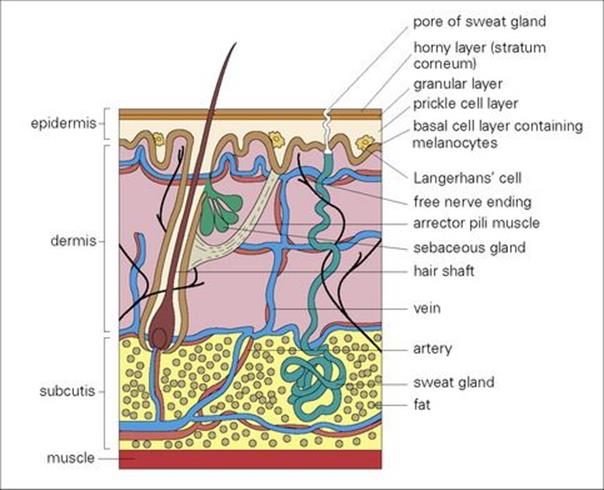
Figure 26.1 Infection of the skin and soft tissue can be related to the anatomy of the skin. Pathogens usually enter the lower layers of the epidermis and dermis only after the skin surface has been damaged.
Microbial disease of the skin may result from any of three lines of attack
These lines of attack are:
• breach of intact skin, allowing infection from the outside
• skin manifestations of systemic infections, which may arise as a result of blood-borne spread from the infected focus to the skin or by direct extension (e.g. draining sinuses from actinomycotic lesions, or necrotizing anaerobic infection from intra-abdominal sepsis)
• toxin-mediated skin damage due to production of a microbial toxin at another site in the body (e.g. scarlet fever, toxic shock syndrome).
The sequence of events in the pathogenesis of mucocutaneous lesions caused by bacterial, fungal and viral infections is outlined in Figure 26.2. Breaches in the skin range from microscopic to major trauma, which may be accidental (e.g. lacerations or burns) or intentional (e.g. surgery). Hospitalized patients are liable to other skin breaches (e.g. pressure sores and intravenous catheter insertions), which may become infected (see Ch. 36). Infections in compromised individuals such as patients with burns are discussed in Chapter 30. Here, we will consider primary infections of the skin and underlying soft tissues, together with mucocutaneous lesions resulting from certain systemic viral infections. Examples of systemic bacterial and fungal infections that cause mucocutaneous lesions are summarized in Table 26.1.
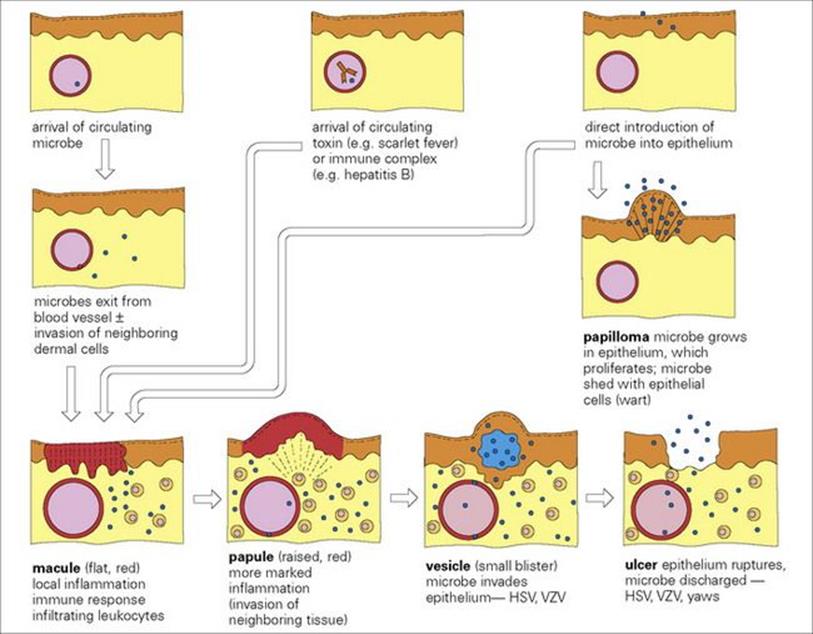
Figure 26.2 The pathogenesis of mucocutaneous lesions. In different infections, the starting point (arrival of microbe or toxin or immune complex) and the final picture (e.g. maculopapular rash, vesicle) will be different. HSV, herpes simplex virus; VZV, varicella-zoster virus.
Table 26.1 Skin manifestations of systemic infections caused by bacteria and fungi
|
Organism |
Disease |
Skin manifestation |
|
Salmonella typhi, Salmonella schottmuelleri |
Enteric fever |
‘Rose spots’ containing bacteria |
|
Neisseria meningitidis |
Septicaemia, meningitis |
Petechial or maculopapular lesions containing bacteria |
|
Pseudomonas aeruginosa |
Septicaemia |
Ecthyma gangrenosum, skin lesion pathognomonic if infected by this organism |
|
Treponema pallidum |
Syphilis |
|
|
Rickettsia prowazekii |
Typhus |
|
|
Streptococcus pyogenes |
Scarlet fever |
Erythematous rash caused by erythrogenic toxin |
|
Staphylococcus aureus |
Toxic shock syndrome |
Rash and desquamation due to toxin |
|
Blastomyces dermatitidis |
Blastomycosis |
Papule or pustule develops into granuloma lesions containing organisms |
|
Cryptococcus neoformans |
Cryptococcosis |
Papule or pustule, usually on face or neck |
Skin lesions are often associated with systemic infection with particular bacteria and fungi. The lesions may provide useful diagnostic aids. Sometimes they are a site from which organisms are shed.
Bacterial infections of skin, soft tissue and muscle
These can be classified on an anatomic basis
The classification depends upon the layers of skin and soft tissue involved, although some infections may involve several components of the soft tissues:
• Abscess formation. Boils and carbuncles are the result of infection and inflammation of the hair follicles in the skin (folliculitis).
• Spreading infections. Impetigo is limited to the epidermis and presents as a bullous, crusted or pustular eruption of the skin. Erysipelas involves the blocking of dermal lymphatics and presents as a well-defined, spreading erythematous inflammation, generally on the face, legs or feet, and often accompanied by pain and fever. If the focus of infection is in the subcutaneous fat, cellulitis, a diffuse form of acute inflammation is the usual presentation.
• Necrotizing infections. Fasciitis describes the inflammatory response to infection of the soft tissue below the dermis. Infection spreads, often with alarming rapidity, along the fascial planes causing disruption of the blood supply. Gangrene or myonecrosis may follow infection associated with ischaemia of the muscle layer. Gas resulting from the fermentative metabolism of anaerobic organisms may be palpable in the tissues (gas gangrene).
The common causative organisms are shown in Table 26.2. Note that the same pathogen (e.g. Streptococcus pyogenes) can cause different infections in different layers of the skin and soft tissue.
Table 26.2 Direct entry into skin of bacteria and fungi
|
Structure involved |
Infection |
Common cause |
|
Keratinized epithelium |
Ringworm |
Dermatophyte fungi (Trichophyton, Epidermophyton and Microsporum) |
|
Epidermis |
Impetigo |
Streptococcus pyogenes and/or Staphylococcus aureus |
|
Dermis |
Erysipelas |
Strep. pyogenes |
|
Hair follicles |
Folliculitis |
|
|
Subcutaneous fat |
Cellulitis |
Strep. pyogenes |
|
Fascia |
Necrotizing fasciitis |
Anaerobes and microaerophiles, usually mixed infections |
|
Muscle |
Myonecrosis gangrene |
Clostridium perfringens (and other clostridia) |
Direct introduction of bacteria or fungi into the skin is the most common route of skin infection. Infections range from mild, often chronic, conditions such as ringworm to acute and life-threatening fasciitis and gangrene. Relatively few species are involved in the common infections.
Staphylococcal skin infections
Staphylococcus aureus is the most common cause of skin infections and provokes an intense inflammatory response
Staphylococcus aureus causes minor skin infections such as boils or abscesses as well as more serious postoperative wound infection. Infection may be acquired by ‘self-inoculation’ from a carrier site (e.g. the nose) or acquired by contact with an exogenous source, usually another person. People who are nasal carriers of virulent Staph. aureus may suffer from recurrent boils, but an inoculum of about 100 000 organisms is thought to be required in the absence of a wound or foreign body. Staph. aureus can also cause serious skin disease due to toxin production (scalded skin syndrome, toxic shock syndrome; see below). In addition, skin and soft tissue infections caused by community-associated, methicillin-resistant Staph. aureus strains (CA-MRSA) are of increasing incidence and concern (see Ch. 36).
A boil begins within 2–4 days of inoculation, as a superficial infection in and around a hair follicle (folliculitis; Fig. 26.3). In this site, the organisms are relatively protected from the host defences, multiply rapidly and spread locally. This provokes an intense inflammatory response with an influx of neutrophils. Fibrin is deposited, and the site is walled off. Abscesses typically contain abundant yellow creamy pus formed by the massive number of organisms and necrotic white cells. They continue to expand slowly, eventually erode the overlying skin, ‘come to a head’ and drain. Drainage inwards can result in seeding of the staphylococci to underlying body sites to cause serious infections such as peritonitis, empyema or meningitis.
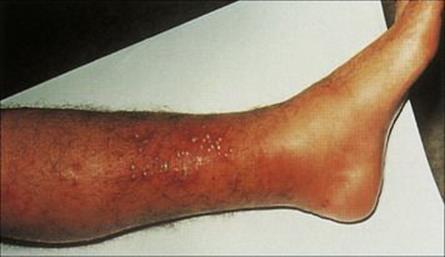
Figure 26.3 Folliculitis. A superficial infection is shown here localized in the hair follicles on the leg. The boils contain creamy-yellow pus and masses of bacteria. Staphylococcus aureus is the most common cause.
(Courtesy of A. du Vivier.)
Staph. aureus infections are often diagnosed clinically and treatment includes drainage and antibiotics
Staph. aureus is the most common cause of boils, and diagnosis is made on clinical grounds. Isolation and further characterization of the infecting staphylococcus in hospital patients and staff are important in the investigation of hospital infections (see Ch. 36).
Treatment involves drainage and this is usually sufficient for minor lesions, but antibiotics may be given in addition when the infection is severe and the patient has a fever. Most Staph. aureus are beta-lactamase producers, but methicillin-susceptible Staph. aureus (MSSA) can be treated with enzyme-stable penicillins such as nafcillin. Isolates resistant to these compounds (i.e. methicillin-resistant Staph. aureus (MRSA); see Ch. 33) may be treated with vancomycin, linezolid, quinopristin-dalfoprisin, or daptomycin. Treatment with these agents does not necessarily eradicate carriage of the staphylococci.
Recurrent infections may be treated in nasal carriers of Staph. aureus with nasal creams containing antibiotics. For example, mupirocin has been used successfully for carriers of methicillin-resistant staphylococci (see Ch. 36). Good skin care and personal hygiene should be encouraged.
Staphylococcal scalded skin syndrome is caused by toxin-producing Staph. aureus
This condition, also known as ‘Ritter’s disease’ in infants and ‘Lyell’s disease’ or ‘toxic epidermal necrolysis’ in older children, occurs sporadically and in outbreaks. It is caused by strains of Staph. aureus producing a toxin known as ‘exfoliatin’ or ‘scalded skin syndrome toxin’. The initial skin lesion may be minor, but the toxin causes destruction of the intercellular connections and separation of the top layer of the epidermis. Large blisters are formed, containing clear fluid, and within 1 or 2 days, the overlying areas of skin are lost (Fig. 26.4), leaving normal skin underneath. The baby is irritable and uncomfortable, but rarely severely ill. However, treatment should take into account the risk of increased loss of fluid from the damaged surface, and fluid replacement may be needed. As mentioned above, antimicrobial chemotherapy would employ beta-lactamase stable penicillins (e.g. nafcillin) against MSSA, whereas vancomycin, linezolid, quinopristin-dalfoprisin, or daptomycin would be used for MRSA.
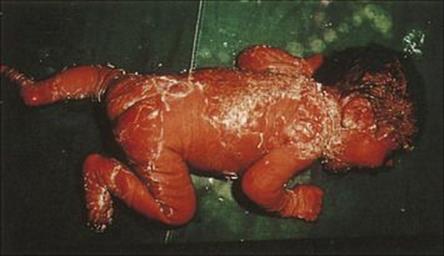
Figure 26.4 Scalded skin syndrome results from infection of the skin with strains of Staphylococcus aureus producing a specific toxin, which destroys the intercellular connections in the skin, resulting in large areas of desquamation. The appearance may be confused with a burn.
(Courtesy of A. du Vivier.)
Toxic shock syndrome is caused by toxic shock syndrome toxin-producing Staph. aureus
This systemic infection came to prominence through its association with tampon use by healthy women, but it is not confined to women and can occur as a result of Staph. aureus infection at non-genital sites (e.g. a wound). Toxic shock syndrome (TSS) involves multiple organ systems and is characterized by fever, hypotension and a diffuse macular erythematous rash followed by desquamation of the skin, particularly on the soles and palms (Fig. 26.5). TSS is caused by exotoxins of Staph. aureus, most commonly TSST1, which behaves as a superantigen (stimulating T-cell proliferation and cytokine release; see Ch. 16). While the prevalence of TSS in the USA is currently estimated at < 200 cases/year, > 90% of adults carry antibodies to TSST1. Treatment of TSS includes steps to open the infected site (e.g. drainage), fluid replacement and antistaphylococcal chemotherapy.
Figure 26.5 Toxic shock syndrome results from systemic infection with Staphylococcus aureus, but has skin manifestations in the form of desquamation, particularly of the palm and soles.
(Courtesy of M.J. Wood.)
Streptococcal skin infections
Streptococcal skin infections are caused by Strep. pyogenes (group A streptococci)
Streptococcal impetigo develops independently of streptococcal upper respiratory tract infection, and although up to 35% of patients carry the same strain in their nose or throat, colonization may well occur after the skin has become infected. The organisms are acquired through contact with other people with infected skin lesions and may first colonize and multiply on normal skin before invasion through minor breaks in the epithelium and the development of lesions. The various risk factors involved in the development of streptococcal impetigo are shown in Figure 26.6. Strep. pyogenes may also cause erysipelas, an acute deeper infection in the dermis. About 5% of patients with erysipelas go on to develop bacteraemia which carries a high mortality if untreated. As discussed previously, impetigo may also be caused by Staph. aureus and occasionally presents in more extreme bullous form (i.e. bullous impetigo) as blisters resembling localized scalded skin syndrome (see above).
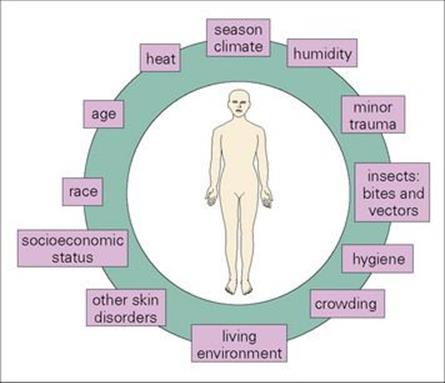
Figure 26.6 Various factors are involved in the development of streptococcal skin infections. Particular M types of Streptococcus pyogenes have a predilection for skin, but various factors predispose the host (usually a child) to infection. Mixed infections with Staphylococcus aureus are also common.
Strep. pyogenes possesses certain surface proteins (M and T) which are antigenic. The species can be subdivided (typed) on the basis of these antigens, and it has been recognized that certain M and T types are associated with skin infection (and these differ from the types associated with sore throats). T proteins play no known role in virulence, and their function is unknown. M proteins are important virulence factors because they inhibit opsonization and confer on the bacterium resistance to phagocytosis. A variety of additional factors contribute to the virulence of the organism, such as lipoteichoic acid (LTA; a component of the Gram-positive cell wall) and F protein, which facilitate binding to epithelial cells.
Clinical features of streptococcal skin infections are typically acute
They develop within 24–48 h of skin invasion and trigger a marked inflammatory response as the host attempts to localize the infection (Fig. 26.7 and Fig. 26.8). Strep. pyogenes elaborates a number of toxic products and enzymes, such as hyaluronidase, which help the organism to spread in tissue. Lymphatic involvement is common, resulting in lymphadenitis and lymphangitis.
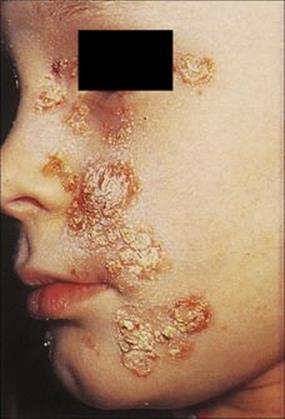
Figure 26.7 Impetigo is a condition limited to the epidermis, with typically yellow, crusted lesions. It is commonly caused by Streptococcus pyogenes either alone or together with Staphylococcus aureus.
(Courtesy of M.J. Wood.)
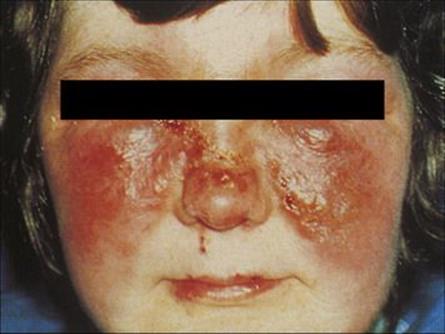
Figure 26.8 Erysipelas. Infection with Streptococcus pyogenes involves the dermal lymphatics and gives rise to a clearly demarcated area of erythema and induration. When the face is involved, there is often a typical ‘butterfly-wing’ rash, as shown here.
(Courtesy of M.J. Wood.)
Lysogenic strains of Strep. pyogenes produce pyrogenic exotoxins (SPE; formally called erythrogenic toxins). As with TSST1 in Staph. aureus (discussed previously), these toxins are superantigens with a potent influence on the immune system. The toxins (e.g. SPEA, B, and C) also act on skin blood vessels to cause the diffuse erythematous rash of scarlet fever, which may occur with streptococcal pharyngitis. Strep. pyogenes may also cause a form of toxic shock syndrome which has been especially associated with the production of the SPEA.
M protein is a major virulence factor in Strep. pyogenes with over 100 types, some of which (e.g. M49) are specifically associated with diseases such as acute glomerulonephritis
Acute glomerulonephritis (AGN) occurs more often after skin infections than after infections of the throat (see Ch. 18). It is characterized by the deposition of immune complexes on the basement membrane of the glomerulus but the precise role of the streptococcus in the causation is still unclear (see Ch. 17); 10–15% of individuals infected with a nephritogenic strain will develop AGN about 2–3 weeks after the primary infection. Most people recover completely, and recurrence after a subsequent streptococcal infection is rare. Rheumatic fever (see Ch. 18) very rarely follows skin infections with Strep. pyogenes.
Streptococcal skin infections are usually diagnosed clinically and treated with penicillin
Gram stains of pus from vesicles in impetigo show Gram-positive cocci, and culture reveals Strep. pyogenes sometimes mixed with Staph. aureus (Fig. 26.9). In erysipelas, skin cultures are often negative, although culture of fluid from the advancing edge of the lesion may be successful.
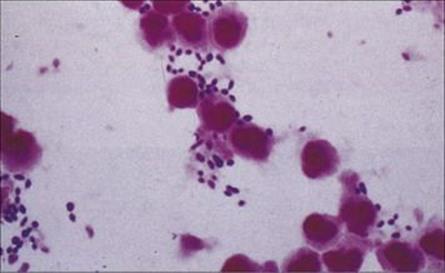
Figure 26.9 Gram-positive cocci in pus.
Penicillin is the drug of choice, although erythromycin, newer macrolides, or an oral cephalosporin may be used for penicillin-allergic patients. However, the prevalence of resistance (e.g. to erythromycin) in streptococci is increasing, and these drugs are not effective in mixed infections withStaph. aureus. Severe infections may require hospitalization.
Impetigo is prevented by improving the host factors associated with acquisition of the disease, as illustrated in Figure 26.6. Since AGN rarely recurs on subsequent streptococcal infection, long-term prophylaxis with penicillin is not indicated (in contrast to the long-term prophylaxis following rheumatic fever; see Ch. 18).
Cellulitis and gangrene
Cellulitis is an acute spreading infection of the skin that involves subcutaneous tissues
Cellulitis extends deeper than erysipelas and usually originates either from superficial skin lesions such as boils or ulcers or following trauma. It is rarely blood-borne, but conversely it may lead to bacterial invasion of the bloodstream. Infection develops within a few hours or days of trauma and quickly produces a hot red swollen lesion (Fig. 26.10). Regional lymph nodes are enlarged and the patient suffers malaise, chills and fever.
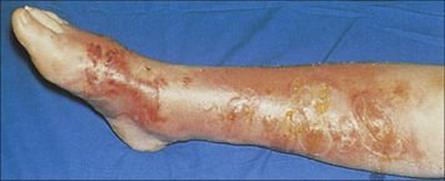
Figure 26.10 When the focus of infection is in the subdermal fat, cellulitis – a severe and rapidly progressive infection – is the typical presentation. Large blisters and scabs may also be present on the skin surface.
(Courtesy of M.J. Wood.)
The great majority of cases of cellulitis are caused by Strep. pyogenes and Staph. aureus. Occasionally, in patients who have had particular environmental exposure, other organisms may be implicated. For example, Erysipelothrix rhusiopathiae is associated with cellulitis in butchers and fishmongers, while Vibrio vulnificus and Vibrio alginolyticus may complicate traumatic wounds acquired in saltwater environments.
The pathogen causing cellulitis is isolated in only 25–35% of cases, and initial therapy should cover streptococci and staphylococci. Attempts can be made to confirm the clinical diagnosis by culture of:
• aspirates from the advancing edge of the cellulitis
• the site of trauma (if present)
• skin biopsies
• blood.
Treatment should be initiated on the basis of the clinical diagnosis because of the rapid progression of the disease, particularly when caused by Strep. pyogenes.
Anaerobic cellulitis may develop in areas of traumatized or devitalized tissue
Such damaged tissue is associated with surgical or traumatic wounds or is found in ischaemic extremities. Diabetic patients are particularly prone to anaerobic cellulitis of their feet (Fig. 26.11). The causative organisms depend upon the circumstances of the trauma: infections in the lower parts of the body are most often caused by organisms from the faecal flora whereas wounds from human bites are infected with oral organisms. Foul-smelling discharge, marked swelling and gas in the tissues are characteristic of anaerobic cellulitis, and a mixture of organisms is usually cultured from the wound. Treatment needs to be aggressive to halt the spread of infection, and both antibiotics and surgical debridement are required. Osteomyelitis (see below) is a common sequela.
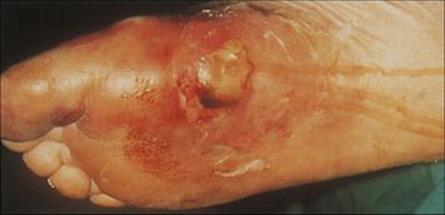
Figure 26.11 Severe progressive cellulitis of the foot. Such cellulitis is usually caused by anaerobic bacteria or a mixture of aerobes and anaerobes and is a particular problem in diabetic patients with peripheral vascular and neuropathic damage.
(Courtesy of J.D. Ward.)
Synergistic bacterial gangrene is a relentlessly destructive infection
This rare infection is caused by a mixture of organisms, typically microaerophilic streptococci and Staph. aureus. The gangrene most commonly follows surgery in the groin or genital area, starting at the site of a drain or suture. Cellulitis develops in the surrounding skin and extends rapidly (within hours), leaving a black necrotic centre. The condition is often fatal, and treatment requires radical excision of the necrotic area and systemic antibiotic therapy.
Necrotizing fasciitis, myonecrosis and gangrene
Necrotizing fasciitis is a frequently fatal mixed infection caused by anaerobes and facultative anaerobes
Although apparently resembling synergistic bacterial gangrene, necrotizing fasciitis is a much more acute and highly toxic infection, causing widespread necrosis and undermining of the surrounding tissues, such that the underlying destruction is more widespread than the skin lesion (Fig. 26.12). Necrotizing fasciitis has been most prominently linked by the popular media with Strep. pyogenes, where it has been frequently termed ‘flesh-eating bacteria’. However, the infection may be caused by a variety of other organisms, especially including MRSA. Patients with necrotizing fasciitis deteriorate rapidly and frequently die. Radical excision of all necrotic fascia is an essential part of therapy, along with antibiotics given both locally to the wound and systemically.
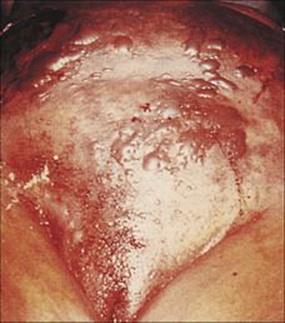
Figure 26.12 Necrotizing fasciitis of the abdominal wall. In patients such as this, infection can be seen rapidly spreading from its origin and causing deep and widespread necrosis. Complete debridement and intensive antimicrobial therapy is required, but the condition is often fatal.
(Courtesy of W.M. Rambo.)
Traumatic or surgical wounds can become infected with Clostridium species
Clostridium tetani gains access to the tissues through trauma to the skin, but the disease it produces is entirely due to the production of a powerful exotoxin (see Ch. 17).
Gas gangrene or clostridial myonecrosis can be caused by several species of clostridia, but Clostridium perfringens is the most common. The organism and its spores are found in the soil and in human and animal faeces, and can therefore gain access to traumatized tissues by contamination from these sources. Infection develops in areas of the body with poor blood supply (anaerobic), and the buttocks and perineum are common sites, particularly in patients with ischaemic vascular disease or peripheral arteriosclerosis. The organisms multiply in the subcutaneous tissues, producing gas and an anaerobic cellulitis, but a characteristic feature of clostridial infection is that the organisms invade deeper into the muscle, where they cause necrosis and produce bubbles of gas, which can be felt in the tissue and sometimes seen in the wound (Fig. 26.13). The infection proceeds very rapidly and causes acute pain. Much of the damage is due to the production by Cl. perfringens of a lecithinase (also known as alpha toxin), which hydrolyses the lipids in cell membranes, resulting in cell lysis and death (Fig. 26.14). The presence of dead and dying tissue further compromises the blood supply, and the organisms multiply and produce more toxin and more damage. Other extracellular enzymes may also play a role in helping the clostridia to spread. If the toxin escapes from the affected area and enters the bloodstream, there is massive haemolysis, renal failure and death.
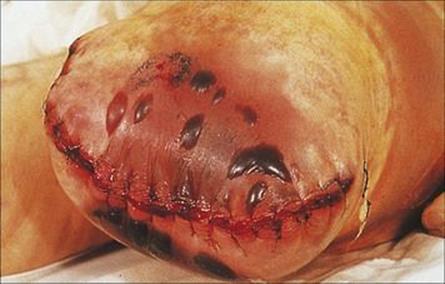
Figure 26.13 Gas gangrene caused by Clostridium perfringens. Organisms from the fecal flora may contaminate a wound and grow and multiply in poorly perfused (anaerobic) tissue. Infection spreads rapidly, and gas can be felt in the tissue and seen on radiographs.
(Courtesy of J. Newman.)
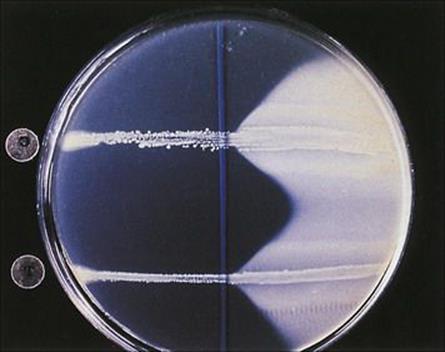
Figure 26.14 The Nagler reaction. Clostridium perfringens produces alpha toxin, which is a lecithinase. If the organism is grown on a medium containing egg yolk (lecithin), enzyme activity can be detected as opacity around the line of growth (right). If anti-alpha toxin is applied to the surface of the plate before inoculation of the organism, the action of the toxin is inhibited (left). This test can be used to confirm the identity of a clostridial isolate.
Amputation may be necessary to prevent further spread of clostridial infection
Because of the rapid progression and fatal outcome of this type of clostridial infection, gangrenous areas require immediate surgery to excise all the affected tissue, and amputation may be necessary. Although some reports suggest that anti-alpha toxin may help if given early enough, antitoxin treatment is not generally viewed as effective, while treatment in a hyperbaric oxygen chamber, where available, may be helpful (i.e. oxygenation of tissue) in some cases.
Antibiotics (e.g. penicillin) are adjuncts to, not replacements for, surgical debridement.
Prevention of infection is of foremost importance. Wounds should be cleansed and debrided early to remove dead and poorly perfused tissue, which the anaerobes favour. Prophylactic antibiotics should be given preoperatively to patients having elective surgery of body sites liable to contamination with faecal flora (see Chs 33 and 36).
Propionibacterium acnes and acne
P. acnes go hand in hand with the hormonal changes of puberty which result in acne
An increased responsiveness to androgenic hormones leads to increased sebum production plus increased keratinization and desquamation in pilosebaceous ducts. Blockage of ducts turns them into sacs in which P. acnes and other members of the normal flora (e.g. micrococci, yeasts, staphylococci) multiply. P. acnes acts on sebum to form fatty acids and peptides which, together with enzymes and other substances released from bacteria and polymorphs, cause the inflammation (Fig. 26.15). Comedones are greasy plugs composed of a mixture of keratin, sebum and bacteria and capped by a layer of melanin (blackheads in popular terminology) (Fig. 26.16).

Figure 26.15 Typical lesions of acne. ‘blackheads’ are seen when plugs of keratin block the pilosebaceous duct.
(Courtesy of A. du Vivier.)
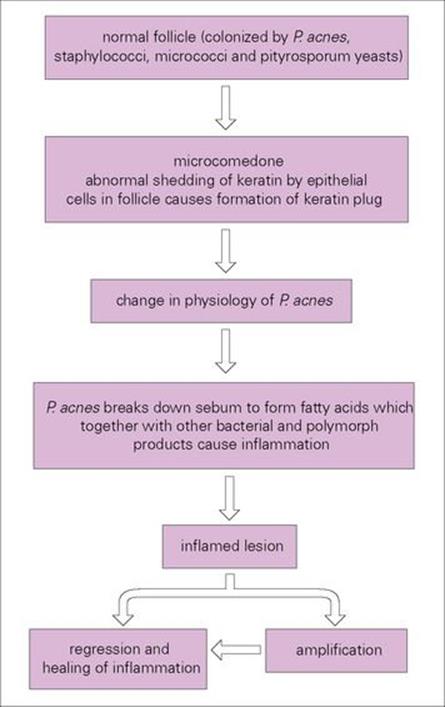
Figure 26.16 The proposed mechanism of the pathogenesis of acne. Hormonal changes in the host initiate the formation of comedones from normal follicles and thereby change the environment of Propionibacterium acnes and its physiologic properties. P. acnes is also known to be an immunostimulator.
Treatment of acne includes long-term administration of oral antibiotics
The antibiotics used to treat acne are usually one of the tetracyclines, or erythromycin. Other treatments include skin care, keratolytics and, in severe cases, synthetic vitamin A derivatives such as isotretinoin. Orally administered antibiotics reduce the surface numbers of P. acnes with a concomitant lowering of the free fatty acids, which act as skin irritants, which result from the activity of bacterial enzymes on sebum. Acne can be a problem for teenagers, but often disappears in older age groups as the sebaceous follicles become less active.
Other Gram-positive rods related to P. acnes, such as corynebacteria and brevibacteria, can also cause skin infections.
Mycobacterial diseases of the skin
Leprosy
Leprosy is decreasing in incidence but still remains a concern
Leprosy has been recognized since biblical times, but in the past, the word was a generic term applied to several different diseases and also implying ‘moral uncleanliness’. Leprosy is thought to have spread to Europe in the sixth century, and by the thirteenth century there were some two hundred leper hospitals in England. Over the centuries that followed, leprosy declined in incidence, and by the fifteenth century was no longer endemic in England; in contrast tuberculosis was on the increase. Now leprosy is rare in the UK and USA and the WHO estimates that the number of new cases worldwide has decreased about 18% per year (2005–2009) with approximately 245 000 new cases detected in 2009. However, the disease still represents a significant problem in SE Asia, Africa and the Americas.
Leprosy is caused by Mycobacterium leprae
Mycobacterium leprae was discovered in 1873 by G.A. Hansen, who identified it as the first bacterial agent capable of causing human disease. Leprosy (Hansen’s disease) appears to be confined to humans. M. leprae is found in nine-banded armadillos, chimpanzees and mangabey monkeys; however, epidemiologic studies have not demonstrated a significant link between this carriage and human disease. Transmission of infection is directly related to overcrowding and poor hygiene and occurs by direct contact and aerosol inhalation. Relatively few organisms are shed from skin lesions, but nasal secretions of patients with lepromatous leprosy are laden with M. leprae. Arthropod vectors may play a role in transmission. Leprosy is not highly contagious, and prolonged exposure to an infected source is necessary; it seems that children living under the same roof as an open case of leprosy are most at risk. Ironically, because the lesions of leprosy are more obvious, patients were in the past excluded from the community and gathered in leper colonies, whereas tuberculosis is much more contagious, but people with tuberculosis were not shunned.
The clinical features of leprosy depend upon the cell-mediated immune response to M. leprae
M. leprae cannot be grown in artificial culture media, and little is known about its mechanism of pathogenicity. Two animal models have been used: infection in the armadillo and in the footpads of mice. The organism grows better at temperatures below 37°C, hence its concentration in the skin and superficial nerves, and it grows extremely slowly; in the mouse footpad the generation time is 11–13 days. Likewise in humans, the incubation period may be many years.
M. leprae grows intracellularly, typically within skin histiocytes and endothelial cells and the Schwann cells of peripheral nerves. The immune response is all important in deciding the type of disease.
M. leprae shares many pathobiologic features with M. tuberculosis, but the clinical manifestations of the diseases are quite different. After an incubation period of several years, the onset of leprosy is gradual and the spectrum of disease activity is very broad depending upon the presence or absence of a cell-mediated immune (CMI) response to M. leprae (Fig. 26.17). At one end of the spectrum is tuberculoid leprosy (TT), characterized by blotchy red lesions with anaesthetic areas on the face, trunk and extremities (Fig. 26.18). There is palpable thickening of the peripheral nerves because the organisms multiply in the nerve sheaths. The local anaesthesia renders the patient prone to repeated trauma and secondary bacterial infection. This disease state is equivalent to secondary tuberculosis (see Ch. 19), with a vigorous CMI response leading to phagocytic destruction of bacteria, and exaggerated allergic responses. TT carries a better prognosis than lepromatous leprosy (LL) and in some patients is self-limiting, but in others may progress across the spectrum towards LL.
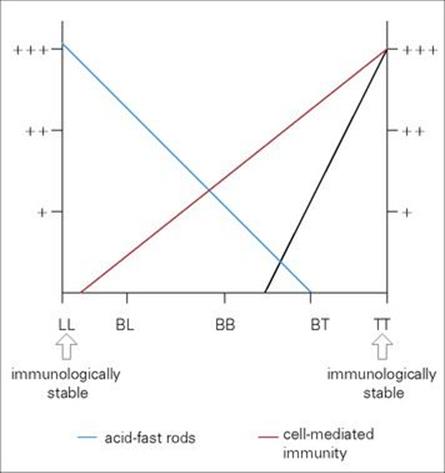
Figure 26.17 Immunologic responses in leprosy. In tuberculoid leprosy (TT) the patient is capable of mounting an effective cell-mediated immune (CMI) response, which makes it possible for macrophages to destroy the organisms and contain the infection. At the other extreme, in lepromatous leprosy (LL) the patient is incapable of producing a CMI response and the organisms multiply unhindered. These patients have many acid-fast rods in their skin and nasal secretions, and are much more infectious than TT patients. Borderline lepromatous (BL), borderline borderline (BB), and borderline tuberculoid (BT) responses are found between these extremes.
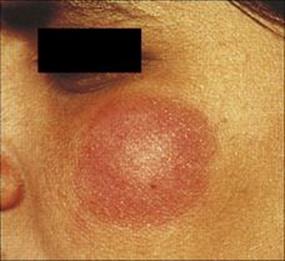
Figure 26.18 Tuberculoid leprosy – a characteristic dry blotchy lesion on the face, but the diagnosis needs to be confirmed by microscopic examination of skin biopsy (see Fig. 26.21).
(Courtesy of the Institute of Dermatology.)
In LL, there is extensive skin involvement with large numbers of bacteria in affected areas. As the disease progresses there is loss of eyebrows, thickening and enlargement of the nostrils, ears and cheeks, resulting in the typical leonine (lion-like) facial appearance (Fig. 26.19). There is progressive destruction of the nasal septum, and the nasal mucosa is loaded with organisms (Fig. 26.20). This form of the disease is equivalent to miliary tuberculosis (see Ch. 19) with a weak CMI response and many extracellular organisms visible in the lesions. The gross deformities characteristic of late disease result primarily from infectious destruction of the nasomaxillary facial structures, and secondarily from pathologic changes in the peripheral nerves predisposing to repeated trauma of the hands and feet and subsequent superinfection with other organisms.
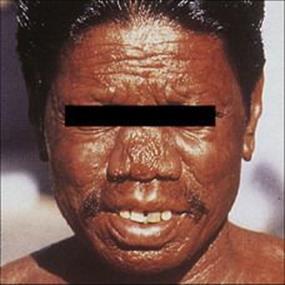
Figure 26.19 Extensive skin involvement in lepromatous leprosy results in a characteristic leonine appearance.
(Courtesy of D.A. Lewis.)
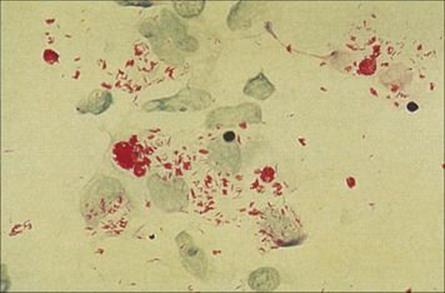
Figure 26.20 In lepromatous leprosy, the nasal mucosa is packed with Mycobacterium leprae, seen here in an acid-fast stain (Ziehl–Neelsen) of nasal scrapings.
(Courtesy of I. Farrell.)
Whether a patient develops TT or LL may in part be genetically determined. Patients with intermediate forms of the disease may progress to either extreme.
M. leprae are seen as acid-fast rods in nasal scrapings and lesion biopsies
Alertness to the possibility of leprosy when confronted with a patient with dermatologic, neurologic or multisystem complaints is of fundamental importance. Although the majority of cases are in people who are not native to Europe or the USA, the diagnosis should also be considered in those who have worked in endemic areas.
Nasal scrapings and biopsies of skin lesions should be stained by Ziehl–Neelsen or auramine stain to demonstrate acid-fast rods. In LL these are numerous, but in TT few if any organisms are seen, but the appearance of granulomas is sufficiently typical to allow the diagnosis to be made (Fig. 26.21). Remember that, in contrast to M. tuberculosis, the organism cannot be grown in vitro.
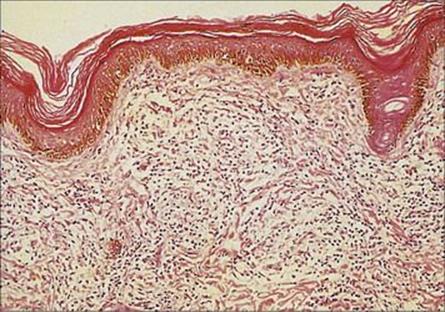
Figure 26.21 In tuberculoid leprosy, the organisms are much sparser but characteristic granulomas form in the dermis, as shown in this histologic preparation.
(Courtesy of C.J. Edwards.)
Treatment
Leprosy is treated with dapsone given as part of a multidrug regimen to avoid resistance
If the disease is diagnosed early and treatment initiated promptly the patient has a much better prognosis. Dapsone (see Ch. 33) had long been the mainstay of therapy, but multidrug therapy is now used because of dapsone resistance:
• For LL, triple therapy with dapsone, rifampin and clofazimine is given for a minimum of 2 years and may be lifelong or until all skin scrapings and biopsies are negative for acid-fast rods.
• For TT, a combination of dapsone and rifampin for 6 months is recommended, the rationale being that in this form of disease there are many fewer organisms and therefore less chance of emergence of resistant mutants.
As a result of multidrug therapy, which is reasonably cheap, well tolerated and effects a complete cure, steady progress is being made towards the elimination of leprosy as a public health problem.
Destruction of the organisms by effective antimicrobial therapy may result in an inflammatory response, erythema nodosum leprosum, which may be severe and, occasionally, fatal. Treatment with corticosteroids or thalidomide may be indicated.
Vaccination with bacille Calmette–Guérin (BCG) has been used in countries with high incidence where potential protection outweighs negative factors such as a positive skin test. Vaccination is not useful for immunocompromised individuals.
Other mycobacterial skin infections
Mycobacterium marinum, M. ulcerans and M. tuberculosis also cause skin lesions
Mycobacterium marinum and M. ulcerans are two slow-growing mycobacterial species that prefer cooler temperatures and cause skin lesions. As its name suggests, M. marinum is associated with water and marine organisms. Human infections follow trauma, often minor such as a graze acquired while climbing out of a swimming pool or while cleaning out an aquarium, which becomes contaminated with mycobacteria from the wet environment. After an incubation period of 2–8 weeks, initial lesions appear as small papules, which enlarge and suppurate and may ulcerate. Histologically, the lesions are granulomas and hence the name ‘swimming pool granuloma’ or ‘fish-tank granuloma’ (Fig. 26.22). Sometimes the nodules follow the course of the draining lymphatic and produce an appearance that may be mistaken for sporotrichosis (see below).
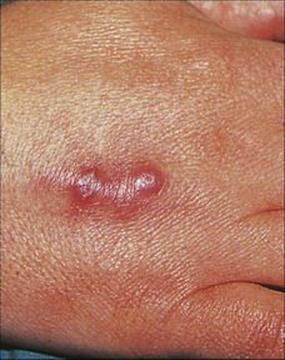
Figure 26.22 Fish-tank granuloma caused by Mycobacterium marinum infection of a lesion acquired while cleaning out a fish tank.
(Courtesy of M.J. Wood.)
M. ulcerans causes chronic, relatively painless cutaneous ulcers known as ‘Buruli ulcers’. This disease is prevalent in Africa and Australia, but is rarely seen elsewhere.
Tuberculosis of the skin is exceedingly uncommon. Infection can occur by direct implantation of M. tuberculosis during trauma to the skin (lupus vulgaris) or may extend to the skin from an infected lymph node (scrofuloderma).
Fungal infections of the skin
Fungal infections may be confined to the very outermost layers of the skin and hair shafts or penetrate into the keratinized layers of the epidermis, nails and hair (the superficial and cutaneous mycoses); others develop in the dermal layers (subcutaneous mycoses). In addition, some systemic fungal infections acquired by the air-borne route have skin manifestations (see Table 26.1).
Superficial and cutaneous mycoses
These are some of the most common infections in humans. Superficial infections of the skin and hair (pityriasis versicolor, tinea nigra, black and white piedras) mainly cause cosmetic problems; cutaneous infections (ringworm, tineas) caused by the dermatophyte fungi are more significant. The important causative agents are the superficial yeast Malassezia furfur and the cutaneous dermatophytes Epidermophyton, Trichophyton and Microsporum.
Pityriasis versicolor
M. furfur is the cause of pityriasis or tinea versicolor
The yeast M. (Pityrosporum) furfur is a common skin inhabitant. The change from commensalism to pathogenicity appears to be associated with the phase change from yeast to hyphal forms of the fungus, but the stimulus for this is unknown. Infections are usually confined to the trunk or proximal parts of the limbs and are associated with hypo- or hyperpigmented macules that coalesce to form scaling plaques. The lesions are not usually itchy and in some patients, they resolve spontaneously.
Malassezia yeasts are also thought to be involved in the pathogenesis of seborrhoeic dermatitis and dandruff.
Diagnosis of pityriasis versicolor can be confirmed by direct microscopy of scrapings
Direct microscopy of scrapings shows characteristic round yeast forms (Fig. 26.23), and treatment with a topical azole antifungal (see below) or with selenium sulphide (2.5%) lotion is appropriate.
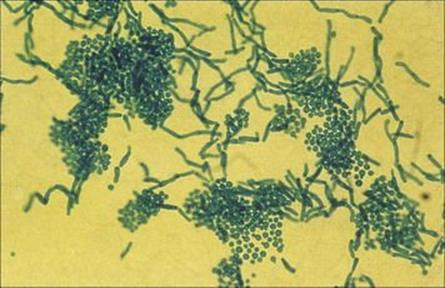
Figure 26.23 Infected skin scales stained to show the thick-walled yeast forms of Malassezia furfur and the short angular hyphae.
(Courtesy of Y. Clayton and G. Midgley.)
Cutaneous dermatophytes
Dermatophyte infections are acquired from many sources and are spread by arthrospores
Species of dermatophytes are described as anthropophilic, zoophilic or geophilic depending upon their primary source (human, animal or soil). The species concerned differ in their geographic distribution, in their predilection for different body sites and in the degree of host response elicited in humans. The source of an infection determines its route of transmission to humans and, to some extent, its distribution in human populations (Fig. 26.24), although population movements are changing established patterns. For example, for a time immigration from Latin America replacedMicrosporum audouinii by Trichophyton tonsurans as the common cause of tinea capitis in the USA, but the latter (which responds poorly to treatment) is now again predominant.
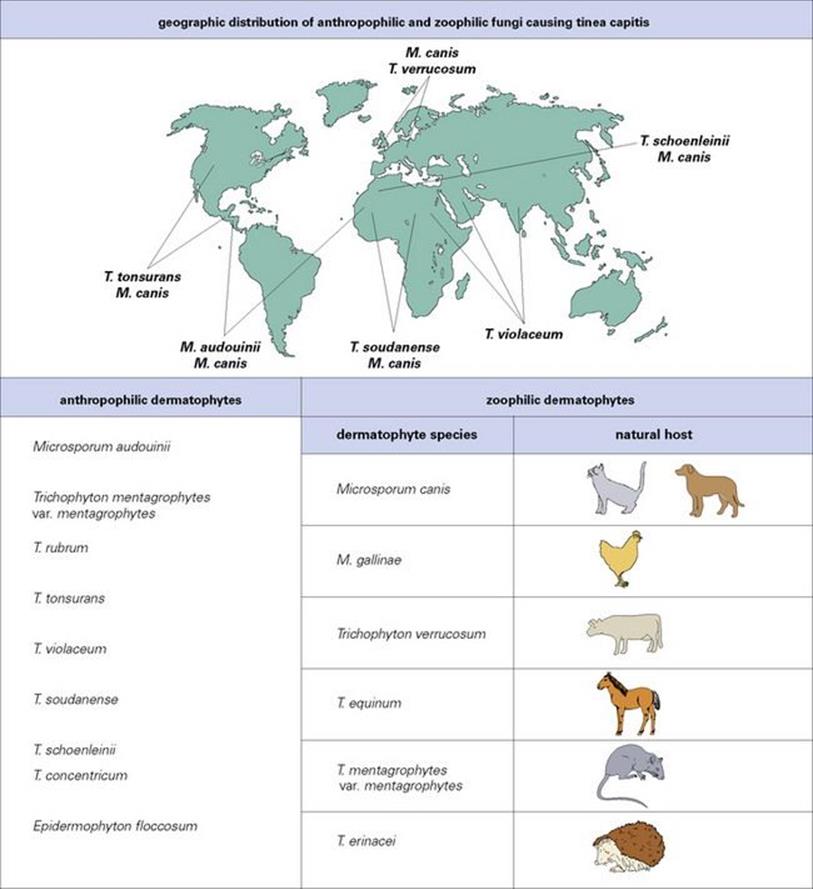
Figure 26.24 Three genera of dermatophytes are important causes of disease: Microsporum, Trichophyton and Epidermophyton. Within each genus there are anthropophilic, zoophilic and geophilic species. The natural host and therefore distribution of anthropophilic species varies.Microsporum gypseum is the geophilic species of importance.
The anthropophilic species are the most common causes of dermatophyte infections. In temperate countries, Trichophyton verrucosum from cattle, T. mentagrophytes from rodents, and Microsporum canis from cats and dogs, are the most common zoophilic causes of human infection. Geophilic species such as Microsporum gypseum are uncommon causes of human disease, but are seen in people who have appropriate exposure, such as gardeners and agricultural workers. Zoophilic and geophilic species tend to cause a greater inflammatory response than anthropophilic species.
Infections are spread by contact with arthrospores, the thick-walled vegetative cells formed by dermatophyte hyphae (Fig. 26.25), which can survive for months. In anthropophilic and zoophilic species, these are shed from the primary host in skin scales and hair.
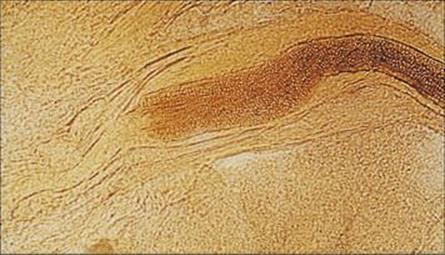
Figure 26.25 Arthrospores of Trichophyton tonsurans in an infected hair shaft. These thick-walled spores are the form in which infection is spread. They can survive in the environment for weeks or months before infecting a new host.
(Courtesy of A.E. Prevost.)
Dermatophytes invade skin, hair and nails
The dermatophytes are keratin-loving organisms and invade the keratinized structures of the body (i.e. skin, hair and nails). The arthrospores adhere to keratinocytes, germinate and invade. The Latin word tinea (meaning a maggot or grub) or ‘ringworm’ is used for these infections because they were originally thought to be caused by a worm-like parasite. Thus, tinea capitis affects the hair and skin of the scalp, tinea corporis the body, tinea cruris the crotch, tinea manuum the hands, tinea unguium the nails and tinea pedis the feet (Fig. 26.26).
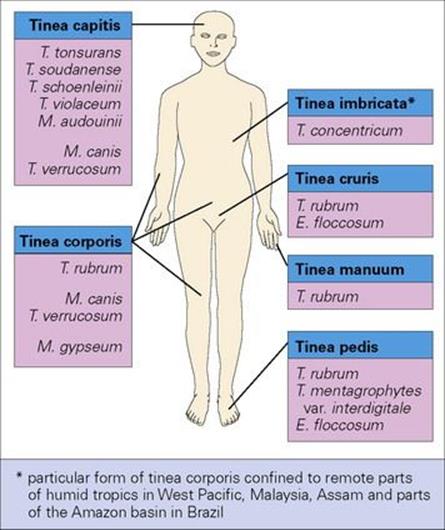
Figure 26.26 Tinea (or ringworm) is the disease of skin, hair and nails caused by dermatophyte fungi. Different species have predilections for different body sites. E., Epidermophyton; M., Microsporum; T., Trichophyton.
The typical lesion is an annular or serpentine scaling patch with a raised margin. The main symptom is itching, but this is variable in degree. The skin is often dry and scaly and sometimes cracks (e.g. between the toes in tinea pedis), while infections of hair cause hair loss (Fig. 26.27). The degree of associated inflammation varies with the infecting species, usually being greater with zoophilic than with anthropophilic species. Individuals also differ in their susceptibility to infection, but the factors determining these differences are not clearly understood. Similarly, dermatophyte species differ in their ability to elicit an immune response; some, such as Trichophyton rubrum, cause chronic or relapsing conditions, whereas other species induce long-term resistance to reinfection. In some patients, circulating fungal antigens give rise to immunologically mediated hypersensitivity phenomena in the skin (e.g. erythema or vesicles) known as dermatophytid reactions. When the skin becomes cracked and macerated as a result of infection, it is liable to superinfection with other organisms such as Gram-negative bacteria in moist sites.
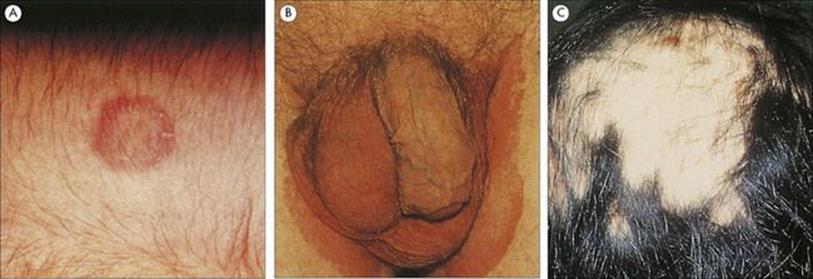
Figure 26.27 (A) Classic annular lesion of tinea corporis, caused here by infection with a Microsporum species. (Courtesy of A.E. Prevost.) (B) Tinea cruris or ‘jock itch’ is a scaly rash on the thighs; the scrotum is usually spared. (Courtesy of M.J. Wood.) (C) Tinea capitis is characterized by scaling on the scalp and hair loss. Some dermatophytes fluoresce under ultraviolet light, and this can be an aid to diagnosis.
(Courtesy of M.H. Winterborn.)
Very rarely, dermatophytes invade the subcutaneous tissues via the lymphatics, causing granulomas, lymphoedema and draining sinuses. Further extension to sites such as the liver and brain may be fatal.
Most dermatophyte species fluoresce under ultraviolet light
This feature can be used as a diagnostic aid, particularly for tinea capitis, in the clinic. Laboratory diagnosis depends upon culture of scrapings or clippings from lesions on Sabouraud agar or other agars to which inhibitory agents (antibiotics/cycloheximide) have been added to provide some selectivity (Fig. 26.28). Dermatophytes infecting hair show a characteristic distribution, which may be helpful for identification:
• Some, such as most Microsporum species, form arthrospores on the outside of the hair shaft (ectothrix infections).
• The majority of Trichophyton infections form arthrospores within the hair shaft (endothrix infection, Fig. 26.29).
Figure 26.28 Dermatophyte infection. Samples of skin, hair and nails need to be ‘cleared’ by treatment with potassium hydroxide before examining under the microscope for the presence of fungal hyphae.
(Courtesy of R.Y. Cartwright.)
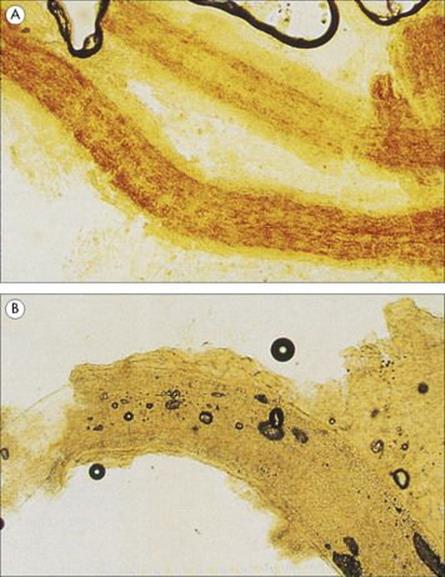
Figure 26.29 Dermatophytes may form arthrospores within the hair shafts (endothrix infection) as shown in (A) and less commonly outside the shaft (ectothrix infection) as shown in (B).
(Courtesy of Y. Clayton and G. Midgley.)
Confirmation of identity depends upon the colonial and microscopic characteristics of the fungi cultured on Sabouraud agar (Fig. 26.30). Growth may take up to 2 weeks, but identification is not difficult and is useful for determining the source of infection.
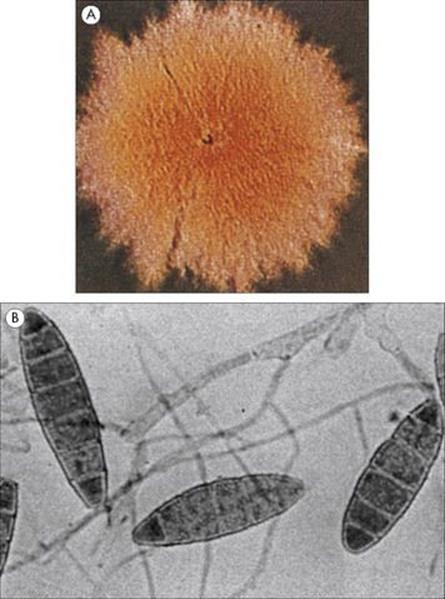
Figure 26.30 (A) Macroscopic growth (colony) and (B) microscopic preparation showing the macroconidia of Microsporum gypseum.
Dermatophyte infections are treated topically if possible
A range of agents is available for topical treatment (see Ch. 33), both antifungals (e.g. miconazole) and keratolytic agents such as Whitfield’s ointment (a mixture of salicylic and benzoic acids). However, infections of nails and hair are better treated by oral antifungal drugs. Terbinafine or itraconazole are now used in preference to griseofulvin. These newer agents may give a cure rate of 70–80% for nail infections.
Candida and the skin
Candida requires moisture for growth
The relative dryness of most areas of skin limits the growth of fungi such as Candida that require moisture. Candida is found in low numbers on healthy intact skin, but rapidly colonizes damaged skin and intertriginous sites (opposed skin sites which are often moist and become chafed, Fig. 26.31). Candida also colonizes the oral and vaginal mucosa and overgrowth may result in disease in these sites (thrush, see Ch. 21). However, a substantial lowering of host resistance (e.g. neutropenia) is necessary for Candida to invade deeper subcutaneous tissue, and disseminated candidiasis does not often originate from skin infection unless there is instrumentation through infected areas (see Ch. 30).
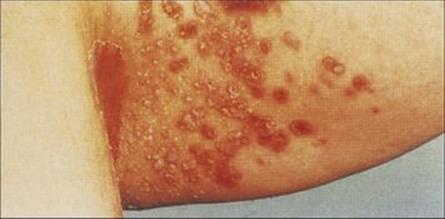
Figure 26.31 Candida infection of the skin. Here, infection has occurred between two apposing skin surfaces, which provide a suitably moist environment for this yeast to multiply.
(Courtesy of A. du Vivier and St Mary’s Hospital.)
Subcutaneous mycoses
Subcutaneous fungal infections can be caused by a number of different species
Lesions usually develop at sites of trauma (a thorn, a bite) where the fungus becomes implanted. With the exception of sporotrichosis, subcutaneous fungal infections are rare, but similar diseases can be caused by certain bacteria such as Actinomyces and atypical mycobacteria, and therefore it is important to establish the aetiology in order to select optimal therapy. The fungi involved are difficult to eradicate with antifungal agents, and surgical intervention, in the form of excision or amputation, is often required.
Sporotrichosis is a nodular condition caused by Sporothrix schenckii
Sporothrix schenckii is a saprophytic fungus that is widespread in nature in soil, on rose and berberis bushes, tree bark and sphagnum moss. Infection is acquired through trauma (e.g. a thorn) and is an occupational hazard for people such as farmers, gardeners and florists. A small papule or subcutaneous nodule develops at the site of trauma 1 week to 6 months after inoculation, and infection spreads, producing a series of secondary nodules along the lymphatics that drain the site (Fig. 26.32). Diagnosis is made by culture of drained or aspirated material onto Sabouraud agar; serological tests are available. Azole drugs are highly effective and, where available, itraconazole or fluconazole have replaced treatment with oral potassium iodide.
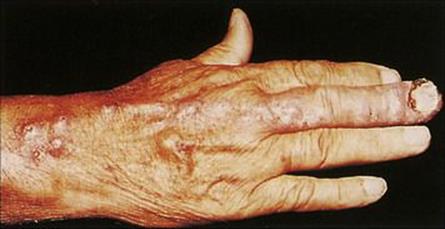
Figure 26.32 Sporotrichosis spreading up the draining lymphatics of the hand following a primary infection in the nailbed of the third finger.
(Courtesy of T.F. Sellers, Jr.)
Disseminated disease can occur following cutaneous or pulmonary infection with S. schenckii. It is more common in compromised patients such as those with underlying carcinoma or sarcoidosis, but many cases occur in people in whom no underlying disease is recognized. Treatment with amphotericin B is indicated, and the prognosis is often poor.
Other species causing subcutaneous infections include Cladosporium and Phialophora (chromoblastomycosis), Pseudallescheria and Madurella (mycetoma).
Systemic fungal infections with skin manifestations include blastomycosis, coccidioidomycosis and cryptococcosis
Skin lesions are the most common presenting symptom of blastomycosis, a disease endemic in Central and North America and Africa and caused by Blastomyces dermatitidis. Infection is acquired by aspiration of the fungal spores and spreads from the primary site in the lung. Blastomycosis can be a systemic disease in apparently immunologically normal hosts (Fig. 26.33). It also causes disease in horses and dogs.
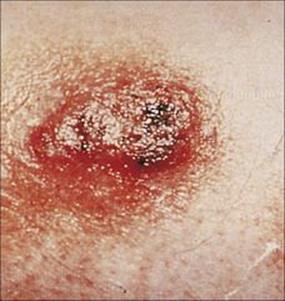
Figure 26.33 Typical skin lesion of blastomycosis. Infection is acquired by the respiratory route, and the primary site of infection is the lung. However, in chronic blastomycosis the skin is the most common extrapulmonary site of infection.
(Courtesy of K.A. Riley.)
Other systemic infections that may have skin manifestations are those caused by Coccidioides immitis and Cryptococcus neoformans.
Parasitic infections of the skin
The skin is a major route of entry for parasites, which may:
• penetrate directly (e.g. schistosomes, hookworm)
• be injected by blood-feeding vectors.
Many of these parasites leave the skin almost immediately as they progress through their life cycle, but some remain there and others may become trapped. A few parasites actually exit from the body through the skin (e.g., release of Guinea worm larvae). Pathologic responses to parasites associated with the skin range from mild to disablingly severe. Some species causing severe conditions are described briefly below.
Leishmaniasis may be cutaneous or mucosal (formerly termed mucocutaneous)
Two major disease complexes caused by the protozoans Leishmania affect the skin and both are transmitted by the bite of sandfly vectors:
• The cutaneous leishmaniases, which occur in both the Old World (Asia, Africa, Southern Europe) and New World (Central and South America), include conditions ranging from localized self-healing ulcers to non-curing, disseminated lesions similar to leprosy in appearance.
• In the New World, mucosal leishmaniasis occurs when the parasite in the skin invades mucosal surfaces (nose, mouth), giving rise to chronic disfiguring conditions. Leishmaniasis is discussed in detail in Chapter 27.
Schistosome infection can cause a dermatitis
Transmission of schistosome infection to humans is achieved by active skin penetration by larvae (cercariae) released into fresh water by the snail intermediate host (see Ch. 27). This stage of infection can give rise to a dermatitis known as swimmers’ itch. It may also be produced by the cercariae of bird schistosomes and is relatively common where natural water used for recreation is populated with aquatic birds. It is a frequent problem in lakes in North America. Topical anti-inflammatory treatments are effective therapy.
Cutaneous larval migrans is characterized by itchy inflammatory hookworm larvae trails
Human hookworms (the nematodes Ancylostoma and Necator) invade the body through the skin, the infective larvae burrowing into the dermis and then migrating via the blood to eventually reach the intestine. Invasion may cause dermatitis (known as ground itch) and this becomes more severe upon repeated infection. Humans, however, can also be invaded by the larvae of the cat and dog species of Ancylostoma. Infection is acquired when exposed skin comes into contact with soil that has been contaminated by animals carrying the adult worms in their intestines. Eggs in the faeces hatch to produce the infective larvae, which remain viable for prolonged periods. As the human host is foreign for these species, the larvae fail to escape from the dermis after invasion, and may live for some time, migrating parallel to the skin and leaving intensely itchy sinuous inflammatory trails (creeping eruption), which are easily visible at the surface (Fig. 26.34). Treatment is with topical thiabendazole paste or oral ivermectin.
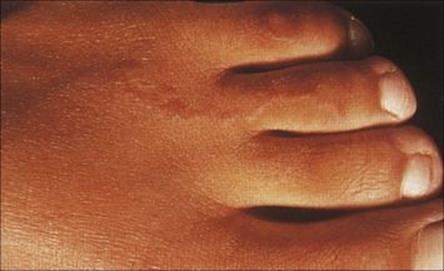
Figure 26.34 Cutaneous larval migrans (creeping eruption), showing the raised inflammatory track left by the invading hookworm larvae.
(Courtesy of A. du Vivier.)
Onchocerciasis is characterized by hypersensitivity responses to larval antigens
Onchocerciasis is also known as river blindness. The adult stages of Onchocerca volvulus live for many years in subcutaneous nodules. Female worms release live microfilariae, which migrate away from the nodules, remaining largely in the dermal layers. They can invade the eye, causing river blindness (see Ch. 25). The slow build-up of parasite numbers and the development of a hypersensitivity response to the antigens released by living and dying larvae give rise to inflammatory skin conditions. In the early stages, these appear as erythematous papular rashes accompanied by intense itching. Later, there is skin thickening, elasticity is lost, and excessive wrinkling occurs and depigmentation is also common. The microfilariae can be killed by ivermectin treatment, but the skin changes, once advanced, are irreversible. Dermal inflammatory condition and secondary bacterial infection are not uncommon during infection with lymphatic filarial nematodes.
Arthropod infections
Some flies, mainly in the tropics and subtropics, have larvae that develop within the skin
Myiasis is a condition associated with invasion of the body by the larvae (maggots) of dipteran flies such as Dermatobia. Several species of fly have a cycle in which the larvae feed and grow in the skin of a mammal, just below the surface, escaping before or after pupation to continue their life cycle and lead ultimately to the release of adult forms. Female flies lay eggs or larvae directly onto the skin, and larvae may then invade wounds or natural orifices. The activities and feeding of the larvae cause intense painful reactions, and large lesions may develop. A number of these species have been found in humans, and infections have been recorded in many countries, although primarily in tropical and subtropical regions. Treatment involves removal of the larvae, alleviation of symptoms and prevention of secondary bacterial infection.
There is a revival of interest in using maggots of non-myiasis species to remove necrotic tissue from wounds, their secretions also preventing bacterial contamination.
Certain ticks, lice and mites live on blood or tissue fluids from humans
Some feed non-selectively on humans, the normal hosts being animals; other species are human specific. The feeding processes and the inevitable release of saliva, give rise to skin irritation, which becomes more intense as the body responds immunologically to the proteins present in the saliva. Prolonged feeding, as practiced by ticks, may leave painful lesions in the skin, which can become secondarily infected. Species such as lice and scabies mites, which spend the greater part or the whole of their lives on the human body, can cause severe skin conditions when populations accumulate. These conditions arise from:
• the activity of the arthropods themselves
• their production of excreta
• the oozing of blood and tissue fluids from the feeding sites
• the host’s inflammatory reaction.
Pediculosis – infection with head and body lice of the genus Pediculus – can, when severe, give rise to encrusting inflammatory masses in which fungal infections may establish. Good personal hygiene prevents infestation; use of insecticidal creams, lotions, shampoos and powders containing malathion or carbaryl helps to clear the insects directly.
The scabies mite has a more intimate contact with the human host than lice, living its whole life in burrows within the skin. The female lays eggs into these burrows, and so the area of infection can spread to cover large areas of the body from the original site, which is usually on the hands or wrists (Fig. 26.35; see Ch. 21). Infection causes a characteristic rash with itching, and secondary infections may follow scratching. Very heavy infections may develop in immunocompromised individuals or in people who are unable to care adequately for themselves. Under these conditions, there is extensive thickening and crusting of the skin (Norwegian scabies). Treatment with permethrin or malathion is recommended; benzyl benzoate can also be used on unbroken skin but is less effective. Oral ivermectin may be required in addition to topical therapy for Norwegian scabies.
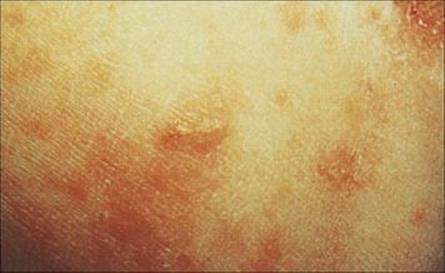
Figure 26.35 A characteristic cutaneous burrow in scabies.
(Courtesy of M.J. Wood.)
Mucocutaneous lesions caused by viruses
Mucocutaneous lesions caused by viruses can be divided into:
• those in which the virus remains restricted to the body surface at the site of initial infection
• those in which the virus causes mucocutaneous lesions after spreading systemically through the body (Table 26.3).
The infections that spread systemically can in turn be divided into:
• those in which the skin lesions (vesicular) are sites of virus replication and are infectious
• those in which the skin lesions (maculopapular) are non-infectious and immunologically mediated, although the virus can be shed from other sites.
The skin rash has a characteristic distribution in many infectious diseases, but with the exception of zoster, the reason for this is unknown.
Table 26.3 Mucocutaneous lesions caused by viruses
|
Virus |
Lesion |
Virus shedding from lesion |
|
No systemic spread |
||
|
Papilloma (wart) |
Common wart; plantar wart; genital wart |
+ |
|
Molluscum contagiosum (poxvirus) |
Fleshy papule |
+ |
|
Orf (poxvirus from sheep, goats) |
Papulovesicular |
+ |
|
Systemic spread |
||
|
Herpes simplex, varicella-zoster |
Vesicular (neural spread and latency) |
+ |
|
Coxsackievirus A (9, 16, 23) |
Vesicular, in mouth (herpangina) |
+ |
|
Coxsackievirus A16 |
Vesicular (hand, foot and mouth disease) |
+ |
|
Parvovirus B19 |
Facial maculopapular (erythema infectiosum) |
− |
|
Human herpesvirus 6 |
Exanthem subitum (roseola infantum) |
− |
|
Measles |
Maculopapular skin rash |
− |
|
Rubella, echoviruses (4, 6, 9, 16) |
Maculopapular not distinguishable clinically |
− |
|
Dengue and other arthropod transmitted viruses |
Maculopapular |
− |
The pathogenesis of these diseases is illustrated in Figure 26.2. Papillomas and vesicular lesions are generally sites of virus shedding. The distribution as well as the nature of the lesion can be important in diagnosis (e.g. varicella), but many maculopapular rashes are clinically indistinguishable.
Rashes are particular features of human infection and are rare in animals. This is because human skin is naked and is a turbulent, highly reactive tissue in which immune and inflammatory events are clearly visible. Rashes cause discomfort and may be painful but they may be very helpful for the clinician who needs to make a diagnosis. The veterinarian is less privileged because the skin of most other mammals is largely covered with fur, and skin lesions generally involve hairless areas such as udders, scrotums, ears, prepuces, teats, noses or paws, which have the human properties of thickness, sensitivity and vascular reactivity.
Papillomavirus infection
Over 120 different types of papillomavirus can infect humans and are species specific
Papillomaviruses are 55 nm in diameter, icosahedral, double-stranded DNA viruses and cause skin papillomas (warts). The 70 different types that can infect humans show < 50% cross-hybridization of DNA, although not all types are common. Human papillomaviruses (HPV) are species specific and distinct from animal papillomaviruses. They are highly adapted to human skin and mucosa and are ancient associates of our species; therefore, for most of the time they cause little or no disease. They show some adaptation to definite sites on the body:
• At least 40 types, including HPV 6, 11, 16 and 18, can infect the anogenital tract and other mucosal areas and are sexually transmitted.
• HPV 1 and 4 tend to cause plantar warts.
• HPV 2, 3 and 10 cause warts on the knees and fingers.
Papillomaviruses are generally transmitted by direct contact, but they are stable and can also be spread indirectly. For instance, plantar warts can be acquired from contaminated floors or from the non-slip surfaces at the edges of swimming pools, and in a given individual warts can be spread from one site to another by shaving.
Papillomavirus infects cells in the basal layers of skin or mucosa and are tissue tropic
After entering the body via surface abrasions, the virus infects cells in the basal layers of the skin or mucosa (see Fig. 26.2). There is no spread to deeper tissues. Virus replication is slow and is critically dependent on the differentiation of host cells. Viral DNA is present in basal cells, but viral antigen and infectious virus are produced only when the cells begin to become squamified and keratinized as they approach the surface. The infected cells are stimulated to divide and finally, 1–6 months after initial infection, the mass of infected cells protrudes from the body surface to form a visible papilloma or wart (Fig. 26.36). There is marked proliferation of prickle cells, and vacuolated cells are present in the more superficial layers. Warts can be:
• filiform with finger-like projections
• flat topped
• flat because they grow inwards due to external pressure (plantar warts)
• a cauliflower-like protuberance (e.g. genital warts)
• a flat area of dysplasia on the cervix.
Immune responses eventually bring virus replication under control and, several months after infection, the wart regresses. Antibodies are demonstrable, but CMI responses are more important in recovery. Viral DNA remains in a latent state in the basal cell layer, infecting an occasional stem cell, and is therefore retained within the layer as epidermal cells differentiate and are shed from the surface. When patients are subsequently immunocompromised (e.g. post-transplant) crops of warts may result from reactivation of latent virus in the skin.
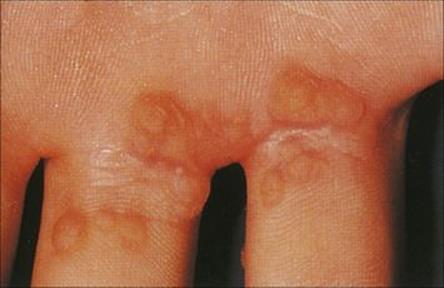
Figure 26.36 Common warts (papillomas) on the hand.
(Courtesy of M.J. Wood.)
Papillomavirus infections are associated with cancer of the cervix, vulva, penis, rectum, head, and neck
Human papillomavirus infections are associated with nearly 4% of all cancers. The association between genital warts and cancer of the cervix, vulva, penis and rectum is referred to in Chapter 17. Infection with specific genital HPVs causes invasive cervical cancer. There is a rare autosomal recessive disease, epidermodysplasia verruciformis, characterized by multiple warts containing many different HPV types which are not normally seen causing skin warts and immunologic defects. Warts may undergo malignant change (squamous cell carcinomas) in nearly 30% of these patients, usually in sun-exposed sites.
Diagnosis of papillomavirus infection is clinical and there are many treatments
Wart viruses cannot be cultivated in the laboratory, and serologic tests are mainly of epidemiological, rather than diagnostic, use. HPV DNA detection methods can be used to examine samples not only for the HPV type but also to quantify the viral load.
Many treatments have been used for warts, some of them doubtless seeming effective because skin warts eventually disappear without treatment. Treatments of skin warts include the application of karyolytic agents such as salicylic acid and destruction of wart tissue by cryotherapy, freezing with dry ice (solid carbon dioxide) or with liquid nitrogen. The latter is the most commonly used and most effective treatment. Genital intraepithelial lesions, especially cervical, can lead to malignant disease, and treatment to eliminate the infection may involve laser therapy, loop excision, and surgery. Immunomodulating and antiviral agents such as imiquimod and topical cidofovir, respectively, have been used in certain clinical settings.
Molluscum contagiosum is an umbilicated lesion caused by a poxvirus
The poxvirus infects epidermal cells to form a fleshy lesion, often with an umbilicated centre (Fig. 26.37). It only infects humans and is spread by contact, or in the case of genital lesions, by sexual intercourse. There are two antigenically distinct types. Poxvirus particles can be seen by electron microscopy (see Ch. 3).
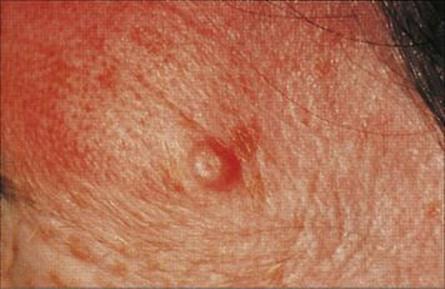
Figure 26.37 Single umbilicated lesion in molluscum contagiosum.
(Courtesy of M.J. Wood.)
Orf is a papulovesicular lesion caused by a poxvirus
Orf (contagious pustular dermatitis) is an uncommon infection of the epidermis and is acquired by direct contact with infected sheep or goats. There is a papulovesicular lesion, generally on the hands, which may ulcerate. It is a clinical diagnosis and can be confirmed by electron microscopy.
Herpes simplex virus infection
Herpes simplex virus infection is universal and occurs in early childhood
Herpes simplex virus (HSV) is a medium-sized (120 nm) double-stranded DNA virus of the herpesvirus group. Two types, HSV-1 and HSV-2, are distinguishable antigenically. They cause a wide variety of clinical syndromes, the basic lesion being an intraepithelial vesicle, from which the virus is shed. Infection is usually transmitted from the saliva or cold sores of other individuals and frequently by kissing.
Clinical features of HSV infection are vesicles and latency
After infection, the virus replicates in cells in the oral mucosa and forms virus-rich vesicles. The patient suffers at most a mild febrile illness. The vesicles ulcerate and become coated with a whitish-grey slough (Fig. 26.38).
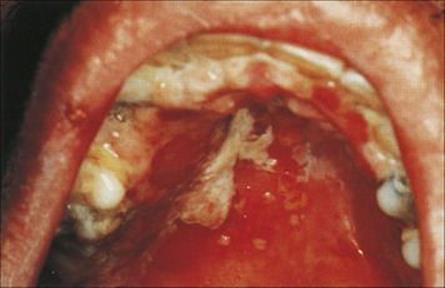
Figure 26.38 Primary herpes simplex virus infection. There are shallow ulcers with white exudate on the palate and gums.
(Courtesy of J.A. Innes.)
During the primary infection, virus particles enter sensory nerve endings in the lesion and are transported to the dorsal root (trigeminal) ganglion, where they initiate latent infection in sensory neurones (see Ch. 16). The lesion resolves as antibody and CMI responses develop. The latent virus remains in the sensory ganglion for life, and under certain circumstances can reactivate and spread down sensory nerves to cause cold sores at the site of the original infection (Fig. 26.39).
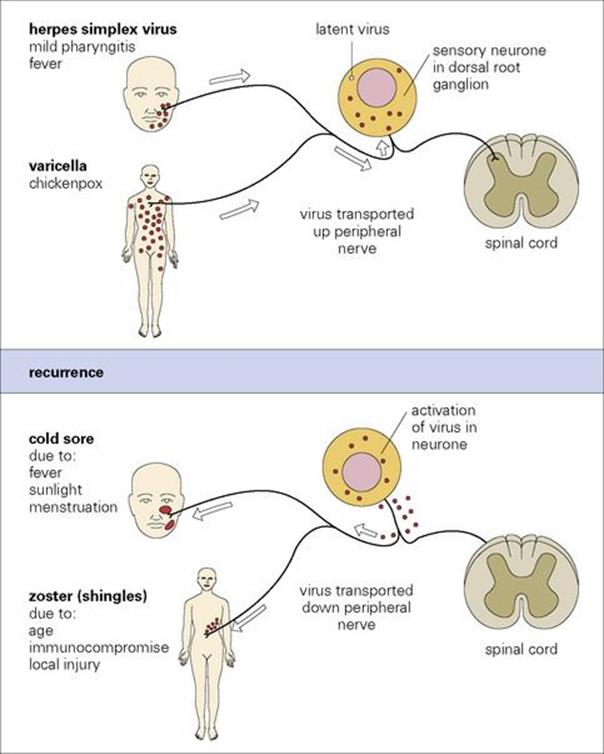
Figure 26.39 Pathogenesis of cold sores and zoster. In both herpes simplex virus and varicella-zoster virus infections the virus in mucocutaneous nerve endings travels up the axon to reach the sensory neurones, where it becomes latent. Recurrences are due to reactivation of the virus within the neurone to become infectious followed by passage of virus down the axon to mucocutaneous site(s) and local spread and replication to form clinical lesion(s).
Primary infection can also occur in:
• the eye, to cause conjunctivitis and keratitis, often with vesicles on the eyelids (see Ch. 25)
• the finger, to cause herpetic whitlow
• other skin sites following direct contact with infected individuals where there is rubbing or trauma, for instance in rugby football (‘scrum pox’) or in wrestlers (‘herpes gladiatorum’)
• the genital tract (see Ch. 21). Although HSV-2 arose as a sexually transmitted variant of HSV-1, the sites infected by the two types are now less clearly distinct.
Serious complications associated with HSV infection include:
• herpetic infection of eczematous skin areas leading to severe disease in young children (Fig. 26.40)
• acute necrotizing encephalitis following either primary infection or reactivation (see Ch. 24)
• neonatal infection acquired from the genital tract of the mother (see Ch. 23)
• primary or reactivating HSV infection in immunocompromised individuals, causing very severe disease (see Ch. 30).
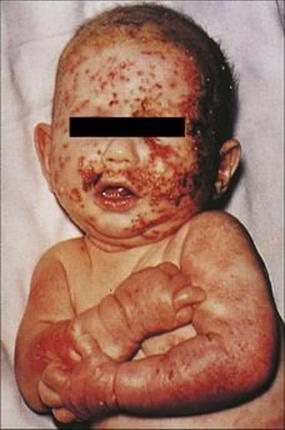
Figure 26.40 Eczema herpeticum due to herpes simplex virus infection in an infant.
(Courtesy of M.J. Wood.)
Herpes simplex virus reactivation is provoked by a variety of factors
In healthy individuals, HSV reactivation is provoked by:
• certain febrile illnesses (e.g. common cold, pneumonia)
• direct sunlight
• stress
• trauma
• menstruation
• immunocompromise.
Reactivation is more severe in immunocompromised patients (see Ch. 30).
A sensory prodrome in the affected area which may include feeling pins and needles, pain, burning, and itching precedes the appearance of the lesion and is due to virus activity in sensory neurones. The lesion, a so-called ‘cold sore’, generally occurs around the mucocutaneous junctions in the nose or mouth (Fig. 26.41). Less commonly, when the ophthalmic branch of the trigeminal ganglion is involved, the lesion is a dendritic ulcer of the cornea. Large amounts of virus are shed in the cold sore, which scabs over and heals over the course of about 1 week. Occasionally, the sensory prodrome occurs without proceeding to a cold sore (see also varicella-zoster virus recurrence below).
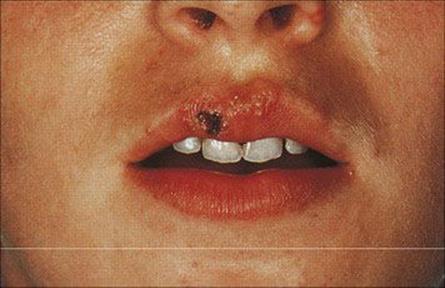
Figure 26.41 Recurrent herpes simplex virus vesicles on the mucocutaneous margin of the lip.
(Courtesy of A. du Vivier.)
Herpes simplex virus is readily isolated from vesicle fluid, and infection is treated with aciclovir
Herpes simplex virus is also readily isolated from saliva, conjunctival fluid and lesions at affected sites. The majority of samples sent to hospital laboratories are from genital lesions, and the virus causes a distinct cytopathic effect when isolated in the laboratory using cell culture lines such as human embryo lung. However, high-sensitivity molecular-based techniques have improved the diagnosis of HSV infection by detecting HSV types 1 and 2 DNA in a variety of samples and have replaced virus culture in many laboratories around the world.
Aciclovir revolutionized the treatment of HSV infection (see Ch. 33) and can be used either topically or systemically. It is relatively non-toxic and acts specifically in virus-infected cells. Recurrent herpetic eruptions have been successfully treated with low doses of aciclovir given twice daily for 6 to 12 months, at which time treatment can be stopped and the frequency of recurrent infection reassessed.
Other antiviral treatment options include valaciclovir and famciclovir. Aciclovir must be given intravenously when treating severe HSV infections such as herpes simplex encephalitis or disseminated HSV infection in immunocompromised individuals. Alternative antivirals such as ganciclovir, foscarnet or cidofovir may be used when antiviral resistance is being considered.
Varicella-zoster virus (VZV) infection
Varicella-zoster virus causes chickenpox (varicella) and zoster (shingles) and is highly infectious
Varicella-zoster virus is a medium-sized (100–200 nm in diameter) double-stranded DNA virus of the herpesvirus group and is morphologically indistinguishable from HSV. There is only one serologic type. The virus grows more slowly than HSV and is not released from the infected cell. Infection is by inhalation of droplets from respiratory secretions and saliva, or by direct contact from skin lesions. Primary infection with VZV causes varicella (chickenpox). Immunity develops and prevents reinfection (a second attack of varicella), but the virus persists in the body, and later in life, after reactivation, causes zoster (shingles). Nearly all humans in resource-rich countries are infected during childhood, but there are many areas of the world where the incidence of chickenpox in children is low, e.g. Africa and the Caribbean islands. The vesicles are an important source of varicella in the community (see Ch. 16).
Varicella is characterized by crops of vesicles that develop into pustules and then scab over
After primary infection, the virus passes across surface epithelium in the respiratory tract to infect mononuclear cells, and is then carried to lymphoid tissues. There are no symptoms and no detectable lesions at the site of entry into the body. The virus slowly replicates in lymphoreticular tissues for about 1 week, and then enters the blood in association with mononuclear cells and is seeded out to epithelial sites. These are mainly the respiratory tract and the skin, but also include the mouth, often the conjunctiva, and probably also the alimentary and urogenital tracts. In the skin, for unknown reasons, the trunk, face and scalp are especially involved. At these epithelial sites, the virus exits from small blood vessels, infecting subepithelial and finally epithelial cells. Multinucleated giant cells with intranuclear inclusions are present in the lesions. In the oropharynx and respiratory tract, the virus reaches the surface and is shed to the exterior to infect other individuals about 2 weeks after initial infection. In the skin, it takes a day or two longer, and it is at this stage, when the characteristic varicella vesicles appear in a centripetal distribution, that a clinical diagnosis can be made (Fig. 26.42) The mean incubation period is 14 days (range 10–23 days).
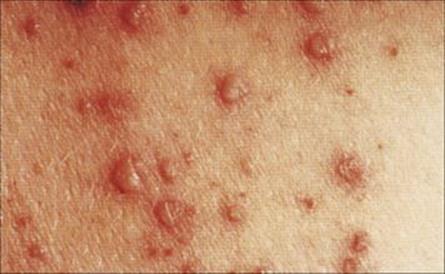
Figure 26.42 Early rash in varicella (chickenpox), with macules, papules and vesicles.
(Courtesy of M.J. Wood.)
The patient remains well until a day or two before the rash, when there may be slight fever and malaise, but the illness is usually mild and often unnoticed. The vesicles appear first on the trunk, then on the face and scalp, and less commonly, on the arms and legs. They often come in ‘crops’ over the course of several days and all stages of lesions occur simultaneously, then develop into pustules, break down, and scab over. The lesions are deeper than with HSV, and scarring is more common. Lesions in the mouth may be painful.
Varicella is usually more severe and more likely to cause complications in adults
The skin lesions of varicella can become infected with staphylococci or streptococci to produce secondary impetigo, but varicella in a child is characteristically a very mild illness. The main complications are:
• interstitial pneumonia, which can be detected radiologically, although it is often subclinical, in up to 20% of adults with varicella; secondary bacterial pneumonia can also occur
• CNS involvement, which may consist of a lymphocytic meningitis or an encephalomyelitis (see Ch. 24).
Thrombocytopenia can occur, but it is usually symptomless. In immunocompromised patients, particularly children with leukaemia, varicella can be a life-threatening disease.
After primary infection during pregnancy the virus may infect the fetus (see Ch. 23), but maternal antibody is present by then and the infection is generally without serious consequences. Congenital varicella syndrome is seen in up to 1–2% if maternal infection occurs in the first or second trimester. Clinical features include skin scarring, hypoplastic limbs, and other stigmata involve the eyes and brain. When the mother is infected a few days before or after delivery, the infant is exposed without the protection of maternal antibody and can suffer a serious disease. Passive immunization with varicella-zoster immune globulin (VZIG) may prevent or attenuate the infection in the infant.
Zoster results from reactivation of latent VZV
During primary infection, VZV in mucocutaneous lesions enters sensory nerve endings and establishes latent infection in sensory neurones in dorsal root ganglia (see Fig. 26.39). Later in life, reactivation can occur to cause zoster in the dermatome, the area of skin supplied by that nerve, at the site of the reactivation. Thoracic dermatomes are most commonly affected because these are the most common sites for the original varicella lesions. Zoster is unilateral because the reactivation is a localized event in a single dorsal root ganglion. Zoster therefore originates from inside the body and is not directly acquired from either varicella or zoster in other individuals. During reactivation in sensory neurones (see Fig. 26.39), there is paraesthesia and pain. Pain may be severe and precedes the development of the erythematous rash in which virus-rich vesicles appear (Fig. 26.43) by several days. It takes a few days for the virus to travel down peripheral nerves and multiply in the skin. Fever and malaise may accompany the rash. Sometimes, the immune response controls the reactivating virus before skin lesions have had time to form, and in this case, the sensory phenomena occur without the skin eruption.
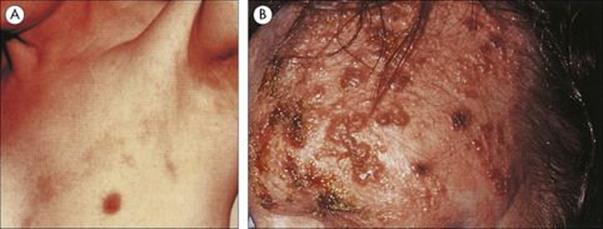
Figure 26.43 Zoster rash. (A) A band of faint erythema, an early sign of shingles, along an intercostal nerve. (B) Rash affecting the ophthalmic division of the trigeminal nerve.
(Courtesy of M.J. Wood.)
Conditions that predispose to zoster include:
• Increasing age. Although zoster is very occasionally seen in childhood, its incidence increases with increasing age, rising from 3/1000 per year in 50–59-year-olds to 10/1000 per year in 80–89-year-olds.
• Immunocompromise due to leukaemia, lymphoma, AIDS, solid organ transplant or other drug-induced immunosuppression.
• Trauma or tumours affecting the brain or spinal cord.
The skin areas affected by zoster reflect the distribution of the original varicella rash, as might be expected from its pathogenesis (see Fig. 26.39). Hence, the trunk is most commonly involved. Ophthalmic zoster involving the upper eyelid, forehead and scalp is a particularly unpleasant manifestation, which can be sight-threatening.
Postherpetic neuralgia is a common complication of zoster
In the healthy host, postherpetic neuralgia (also known as zoster-associated pain, ZAP) is common, especially in the elderly. The pain, which can be severe early in the illness, continues for up to several months after the lesions have resolved. It is difficult to treat, although antiviral agents reduce the incidence, duration and severity of ZAP if started as soon as possible after zoster occurs.
Zoster may be severe in immunocompromised patients. A few days after the localized eruption, the virus, with inadequate control by cell-mediated immunity, spreads via the blood to produce skin and visceral lesions throughout the body. Haemorrhagic complications and pneumonia may occur.
Laboratory diagnosis of VZV
It is a clinical diagnosis that can be assisted by carrying out molecular tests to detect VZV DNA in swabs from the vesicles or alternative tests less widely used including immunofluorescence tests on skin lesion scrapings using VZV-specific monoclonal antibodies or by isolating VZV in cell culture, although the cytopathic effect may take a couple of weeks. Herpesvirus particles can be seen by electron microscopy in vesicle fluid, but are indistinguishable from other herpesviruses, and in particular HSV, which also causes vesicular lesions. Past infection is determined by detecting VZV IgG by enzyme-linked immunosorbent assay (ELISA) or other methods. A VZV IgM result may be helpful if the skin lesions have healed and the diagnosis needs to be made for clinical reasons.
Treatment of varicella and zoster infection
Varicella-zoster virus is much less sensitive than HSV to acyclovir, but this drug, or the more readily bioavailable valaciclovir or famciclovir, can be used orally to treat varicella and zoster. Treatment is often not considered, as chickenpox is thought of as a mild infection that causes little discomfort. However, varicella can cause complications in adolescents and adults, and antiviral treatment should be offered, especially as new lesion formation, viral shedding and symptoms will be reduced. Severe infections must be treated with intravenous acyclovir, especially in high-risk groups. VZIG contains a high titre of human antibody to the virus, and is used to prevent varicella in susceptible individuals at risk of complications (e.g. immunocompromised patients) after exposure. Varicella skin lesions may be treated with calamine lotion to relieve itching and to prevent scratching and secondary infection.
A live attenuated vaccine is licensed in a number of countries, and universal childhood immunization was started in the USA in 1995.
Rashes caused by coxsackieviruses and echoviruses
Coxsackieviruses and echoviruses cause a variety of exanthems (skin rashes)
Sometimes, such infections are accompanied by an enanthem (lesions on internal epithelial surfaces such as the oral cavity). These infections are generally seen in young children, are not usually distinguishable on clinical examination, and are not severe. These viruses are also responsible for illnesses affecting the CNS (see Ch. 24), the upper respiratory tract (see Ch. 18), and striated and heart muscle (see below).
Coxsackievirus A lesions are usually vesicular and occur mostly on the buccal mucosa and the tongue. Most children complain of a sore mouth or tongue and there is slight fever. When vesicular lesions are also seen on the skin, principally on the hands and feet, the condition is called ‘hand, foot and mouth disease’ (Fig. 26.44). The virus is present in the lesions, and coxsackievirus A16 is the most common cause.
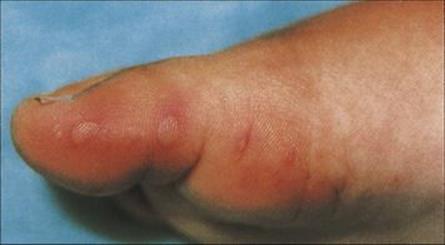
Figure 26.44 Vesicular lesions on the foot in hand, foot and mouth disease.
(Courtesy of M.J. Wood.)
Maculopapular rashes resembling rubella and often occurring in the summer are common manifestations of some coxsackie A and echovirus infections.
Rashes caused by human parvovirus B19
Parvovirus B19 causes slapped cheek syndrome
Parvoviruses are very small (22 nm in diameter) single-stranded DNA viruses. The defective parvoviruses require a helper (adeno-) virus to replicate, and are called ‘adeno-associated viruses’; there are four serotypes and infection is common, but they have not been implicated in any human disease. However, parvovirus B19 grows in mitotically active cells and causes a febrile illness in children with a characteristic maculopapular rash on the face (‘slapped cheek syndrome’). The condition is referred to as ‘erythema infectiosum’ and sometimes ‘fifth disease’, it being the fifth of the six common exanthematous infections recognized by nineteenth-century physicians.
Symptomless parvovirus B19 infection is common and spreads by respiratory droplets
Nearly 50% of the population has had parvovirus B19 in the past. The virus grows in haemopoietic cells in the bone marrow, and although this normally causes no more than a temporary and barely detectable fall in haemoglobin levels, it can lead to serious consequences in those with chronic anaemia. In children with sickle cell anaemia, for instance, the effect on erythropoiesis may cause an aplastic crisis. The virus can also cause arthralgia when it infects adults. Laboratory diagnosis is made by testing sera for parvovirus B19-specific IgM. Molecular tests may be used to detect B19 DNA in fetal blood when hydrops fetalis is suspected. Parvovirus B19 cannot be isolated in cell culture.
Rashes caused by human herpesviruses 6 and 7 (HHV-6 and -7)
Human herpesviruses-6 is present in the saliva of over 85% of adults and causes roseola infantum
Human herpesviruses-6 and -7 were discovered in 1986 and 1990, respectively, the preceding five HHVs being HSV-1, HSV-2, VZV, CMV and EBV. Both infections are prevalent globally and occur in most of the population in the first 2 years of life. HHV-6 replicates in T and B cells and also in the oropharynx, from where it is shed into saliva. The virus persists in the body after initial infection. There are two HHV-6 variants, namely HHV-6A and 6B. Their tissue distribution differs in that HHV-6B can be detected in blood, saliva and in brain tissue whereas 6A occurs more often in the lungs and skin.
HHV-6B is the cause of exanthem subitum (also called roseola infantum), a very common acute febrile illness in infants and young children. After an incubation period of about 2 weeks, children develop a high fever which lasts for 3–5 days. The disease is mild, and within 2 days of the fever subsiding a maculopapular rash is seen (Fig. 26.45). HHV-6B is also associated with about 30% of febrile fits in children and HHV-6 encephalitis has been reported in bone marrow transplant recipients.
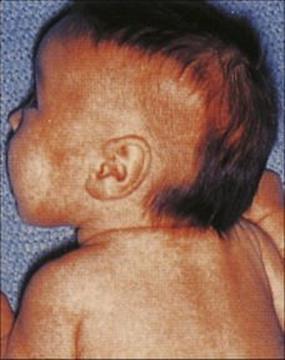
Figure 26.45 Maculopapular rash in roseola infantum.
(Courtesy of M.J. Wood.)
Human herpesviruses -7 is acquired slightly later in early childhood
Human herpesviruses-7 has been isolated from CD4-positive T cells. Infection occurs later than HHV-6 during infancy and early childhood. Persistence in the saliva and exanthem subitum have been reported with HHV-7.
Human herpesviruses-8 is associated with all forms of Kaposi’s sarcoma skin lesions
After a number of epidemiologic reports, it had been thought that a transmissible agent was involved in the development of Kaposi’s sarcoma (KS), a skin malignancy more common in some Mediterranean areas and parts of Africa in addition to AIDS-associated KS. A number of molecular technology developments allowed the identification of KS-associated herpesvirus (KSHV), also known as HHV-8, in 1994 in the endothelial cells of KS lesions. It is not a ubiquitous infection and has also been associated with two other rare malignancies, primary effusion lymphoma and multicentric Castleman’s disease. Transmission is mostly via saliva, and interactions between defective cellular immune responses, the endothelial system and KSHV result in the pathogenesis of KS.
It is a clinical diagnosis. The incidence of AIDS-associated KS has dropped since the advent of highly active antiretroviral therapy. In addition, retrospective studies have shown that ganciclovir and foscarnet treatment has resulted in a reduction of KS lesions.
Smallpox
Smallpox (variola) was a major scourge of humankind for at least 3000 years. It was caused by a poxvirus and spread from person to person by contact with skin lesions and via the respiratory tract. The disease was severe, with a generalized rash (Fig. 26.46), and was fatal in up to 40% of cases, depending upon the strain of virus.

Figure 26.46 Smallpox. A–C These pictures were used as smallpox recognition cards by the World Health Organization during its smallpox eradication campaign. After upper respiratory tract infection, the virus reached the skin, where it replicated to cause a widespread vesiculopustular rash, with later scarring, especially on the face. The fatality rate was up to 40%, depending on the age of the host and the strain of the virus.
(Courtesy of the World Health Organization.)
Global smallpox eradication was officially certified in December 1979
During the first part of the twentieth century, smallpox was largely eradicated from Oceania, North America and Europe by widespread vaccination, as originally developed by Edward Jenner (see Ch. 34), using a live attenuated strain of virus (vaccinia virus), together with strict controls at frontiers. In 1967, the WHO started a campaign to eradicate smallpox from the world, focusing on South America, Africa, India and Indonesia, making use of vaccination, surveillance and containment of cases. Despite such daunting difficulties as cultural barriers, warfare and transport to remote areas, the campaign was successful. Occasional cases had continued to occur in the USA until the 1940s, and in 1974, there were 218 000 cases worldwide, mostly in Asia, but the last case was recorded in Somalia in October 1977. The total cost to the WHO was about US$150 million.
Global eradication of smallpox was possible for a variety of reasons
These reasons are as follows:
• There were no subclinical infections, so cases could be readily identified.
• The virus was eliminated from the body on recovery, with no carriers.
• Humans were the only host (no animal reservoir).
• An effective vaccine was available.
For a few years there were concerns about monkeypox, a simian disease caused by a similar virus and acquired by contact with infected monkeys in Africa. It is, however, poorly transmitted from human to human. However, over 80 people were believed to have contracted monkeypox in the USA in 2003. This was thought to be due to contact with infected prairie dogs. Concerns about the use of smallpox by bioterrorists have resulted in countries making contingency plans for the potential threat, which include the stockpiling of smallpox vaccine.
Measles
Measles has several special features, as follows:
• Nearly all infected individuals become unwell and develop disease. This is in contrast to most other viral infections, in which a significant proportion of individuals undergo an asymptomatic or subclinical infection.
• The disease is so characteristic that a clinical diagnosis can nearly always be made without the need for laboratory help. We can recognize measles as described 1000 years ago by the Arabian physician, Rhazes.
• There is only one antigenic type of measles virus.
• After infection, there is complete resistance to reinfection, which is probably lifelong. Second attacks are almost unknown.
• Measles is highly infectious, and nearly all susceptible children contract the disease on exposure. Until recently, measles was regarded as a routine inescapable part of childhood, and more than 99% of individuals were infected until immunization programmes were developed.
• There is a striking contrast between measles in well-nourished children with good access to medical care (i.e. in resource-rich countries) and measles under conditions of malnutrition or starvation or with poor medical services (i.e. resource-poor countries, Table 26.4).
Table 26.4 The clinical impact of measles depends upon the host
|
Site of virus growth |
Well-nourished child; good medical care |
Malnourished child; poor medical care |
|
Lung |
Temporary respiratory illness |
Life-threatening pneumonia |
|
Ear |
Otitis media quite common |
Otitis media more common, more severe |
|
Oral mucosa |
Koplik’s spots |
Severe ulcerating lesions |
|
Conjunctiva |
Conjunctivitis |
Severe corneal lesions, secondary bacterial infection, blindness may result |
|
Skin |
Maculopapular rash |
Haemorrhagic rashes may occur (‘black measles’) |
|
Intestinal tract |
No lesions |
Diarrhea – exacerbates malnutrition, halts growth, impairs recovery |
|
Urinary tract |
Virus detectable in urine |
No known complications |
|
Overall impact |
Serious disease in a small proportion of those infected |
Major cause of death in childhood (estimated 1 million deaths/year worldwide) |
Measles is a much more serious disease in malnourished children with poor access to medical care. The same epithelial surfaces are infected more extensively and with more serious sequelae.
Aetiology and transmission
Measles outbreaks occur every few years in unvaccinated populations
The basic virology of this paramyxovirus is described in Chapter 3 and it is transmitted by respiratory droplets. Although the virus is soon inactivated as it dries on surfaces, it is more stable in droplets suspended in the air. In unvaccinated populations, outbreaks tend to occur every few years when the number of susceptible children reaches a high enough level. There were a number of measles outbreaks in Europe in 2011, some of which were large, including more than 7000 cases in France, that resulted in further immunization campaigns in a number of countries.
Clinical features of measles include respiratory symptoms, Koplik’s spots and a rash
The inhaled virus enters the body at unknown sites in the upper or lower respiratory tract or conjunctiva and spreads to subepithelial and local lymphatic tissues, without causing detectable lesions or symptoms. During the next few days, there is a primary viraemia and the virus slowly spreads and multiplies in lymphoid tissues elsewhere in the body, including the spleen and the respiratory tract. There is then a secondary viraemia around 5 days after the initial infection and the virus disseminates to a variety of epithelial sites including the skin, kidney, and bladder. Clinical signs soon appear in the respiratory tract where there are only one or two layers of epithelial cells to traverse. The patient is well until 9–10 days after infection and then develops an acute respiratory illness with a runny nose, fever and cough. Conjunctivitis is also a feature, and as a result of the large amounts of virus being shed in respiratory secretions, the patient is highly infectious. The diagnosis may be suspected during this prodromal illness, especially after known exposure to measles. It takes a day or two longer for the foci of infection at mucosal and skin surfaces to cause lesions. Koplik’s spots, pathognomonic of measles, appear inside the cheek (Fig. 26.47), and shortly afterwards the maculopapular rash (Fig. 26.48) is seen, first on the face, then spreading down the body to the extremities.
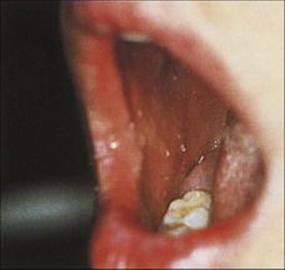
Figure 26.47 Koplik’s spots seen as minute white dots on the inflamed buccal mucosa of a patient with measles.
(Courtesy of M.J. Wood.)
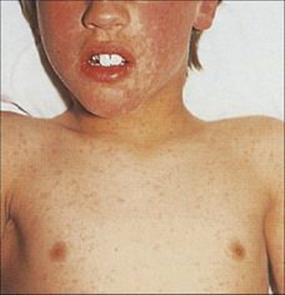
Figure 26.48 Maculopapular rash on the face and trunk of a patient with measles.
(Courtesy of M.J. Wood.)
Measles rash results from a cell-mediated immune response
Antibodies are formed, but a cell-mediated immune (CMI) response is needed to control the virus in the lungs and elsewhere. Without it, the virus continues to multiply and gives rise to giant cell pneumonia (see Ch. 19). The CMI response is also responsible for the skin lesions, which are not seen in patients with serious defects in this type of immunity. Children with agammaglobulinaemia, on the other hand, have a normal course of disease, develop normal immunity, and can be protected by vaccination. In uncomplicated cases, recovery is rapid.
During measles, as in a variety of other acute infections, there are temporary defects in immune responses to unrelated antigens. For instance, at about the time the rash appears, individuals who are known to be tuberculin-positive give negative skin test responses to tuberculin. This returns to normal in about 1 month. During the ‘virgin-soil’ epidemic, when measles reappeared after a long absence in Southern Greenland, in 1953, and adults as well as children were infected, there was increased mortality in those previously infected with tuberculosis.
Complications of measles are particularly likely among children in resource-poor countries
Complications of measles include:
• opportunistic bacterial superinfections, which are quite common, especially otitis media and pneumonia, as a result of virus damage to respiratory surfaces
• a primary measles virus pneumonia (giant cell pneumonia), which is seen in patients with serious CMI response defects
• encephalitis, which occurs in about 1 in 1000 cases (see Ch. 24)
• very rarely, subacute sclerosing panencephalitis (SSPE). This develops 1–10 years after apparent recovery from acute infection.
Children in countries where there is poor medical care and malnutrition develop a more serious disease (see Table 26.4), especially during famine. This is attributable to:
• poor local mucosal defences, which can be improved by vitamin A administration
• impaired immune defences due to protein–calorie malnutrition, with the added impact of measles virus-induced immunosuppression
• poor medical services, with less ready availability of antibiotics to control secondary infection
• high levels of bacterial contamination of the environment
• exposure to a larger virus dose – a possible factor if others with severe measles shed larger amounts of virus from the respiratory tract.
Diagnosis, treatment and prevention
Measles is usually diagnosed clinically; ribavirin can be used as antiviral treatment if clinically indicated and there is a safe and effective vaccine
Although the clinical diagnosis should be clear, the rash is similar to a number of other viral exanthems which affect the same age group. In addition, with the success of the vaccine, the incidence of measles infection has fallen and it is less likely that healthcare workers will see children with measles in resource-rich countries. Therefore, the measles virus RNA or IgM assay is helpful in confirming the diagnosis either on blood or saliva samples. Virus isolation in cell culture is rarely necessary. Complicated measles infection can be treated with ribavirin.
A live attenuated vaccine has been available since 1963. It is effective, safe and long-lasting, and is combined with mumps and rubella vaccines (MMR vaccine; see Ch. 34). Before a vaccine became available, measles killed 7–8 million children each year worldwide. By 1996, this was reduced to 1 million, and the WHO/UNICEF initiatives included applying the mass immunization programmes used in the Americas and Europe to resource-poor countries.
Rubella
Rubella virus infection causes a multisystem infection, but its main impact is on the fetus
There is only one serotype of this single-stranded RNA togavirus, and its principal impact is on the fetus (see Ch. 23). It is transmitted by droplet infection, and is less contagious than measles, but more so than mumps.
After entering the body via the upper respiratory tract, the virus replicates for a period in local lymphoid tissues, followed by spread to the spleen and to lymph nodes elsewhere in the body. One week after infection further multiplication in these tissues leads to viraemia and localization of virus in the respiratory tract and skin, and sometimes the placenta, joints and kidney. The pathogenesis of rubella is outlined in Figure 26.49, and the clinical consequences of infection in various tissues of the body are shown in Table 26.5.
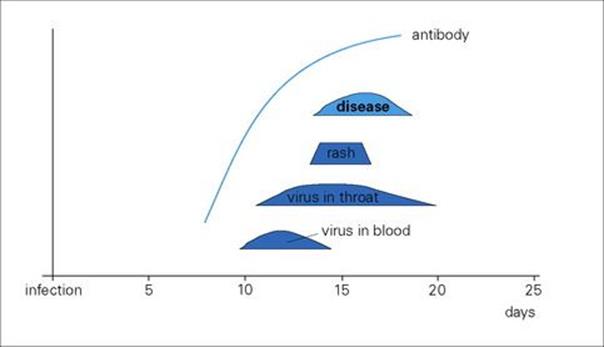
Figure 26.49 The pathogenesis of rubella. Rubella is generally a very mild, often subclinical infection, but it can cause arthritis and has a major impact when it infects the fetus.
Table 26.5 Clinical consequences of rubella virus invasion of different body tissues
|
Site of virus growth |
Result |
Comment |
|
Respiratory tract |
Virus shedding but symptoms minimal (mild sore throat, coryza, cough) |
Patient infectious 5 days before to 3 days after symptoms |
|
Skin |
Rash |
Often fleeting, atypical; immunopathology involved (Ag-Ab complexes) |
|
Lymph nodes |
Lymphadenopathy |
More common in posterior triangle of neck or behind ear |
|
Joints |
Mild arthralgia, arthritis |
Immunopathology involved (circulating immune complexes) |
|
Placenta/fetus |
Placentitis, fetal damage |
Congenital rubella |
After an incubation period of 14–21 days, there is a mild disease, with fever, malaise and an irregular maculopapular rash lasting 3 days. Enlarged lymph nodes are often evident behind the ear, but the infection is commonly subclinical.
Rubella is diagnosed serologically; there is no treatment, but there is a vaccine
Clinical diagnosis of rubella is sometimes possible but should be confirmed in the laboratory. Laboratory diagnosis is made by demonstrating rubella-specific IgM antibodies (see Ch. 32). Virus isolation from the throat is rarely indicated – virus isolation requires specialized cell lines, and indirect methods are needed to demonstrate its growth. Viral RNA can be detected in samples from different sites.
There is no antiviral treatment. A live attenuated rubella vaccine that is safe and effective is given by injection, generally in combination with measles and mumps vaccines (MMR vaccine). Prevention of congenital rubella is referred to in Chapter 23.
The maculopapular rashes seen in certain arthropod-borne virus infections (e.g. dengue) and in zoonotic virus infections (e.g. Marburg disease) are referred to in Chapters 27 and 28. A maculopapular rash may be seen in the prodromal stage of hepatitis B virus infection (see Ch. 22) and is immune complex mediated.
Other infections producing skin lesions
Other bacterial, fungal and rickettsial infections produce a variety of rashes or other skin lesions
Most of these are referred to elsewhere in this book, and they are listed in Table 26.1. Rashes in rickettsial infections are often striking, as in the case of Rocky Mountain spotted fever or typhus (see Ch. 27). Most rickettsia invade vascular endothelial cells and are shed into the blood to infect blood-sucking arthropod vectors. Invasion of vascular endothelial cells in the skin provides the basis for the skin rash but is not a source of direct shedding to the exterior.
Kawasaki syndrome
Kawasaki syndrome is an acute vasculitis and is probably caused by superantigen toxins
The Kawasaki syndrome is a childhood illness that occurs in genetically susceptible hosts with dysregulated T-cell activation after exposure to infectious triggers. Patients, who are generally under 4 years of age, develop fever, conjunctivitis and a rash. There is dryness and redness of the lips and red palms and soles with some oedema, desquamation of fingertips, often arthralgia and myocarditis, which gives a case mortality of about 2%. The basic pathology is an acute multisystemic vasculitis, and 20% of untreated patients develop coronary artery aneurysms. The disease is more common in those of Asian ethnicity, but occurs worldwide. There is no clear evidence for person-to-person transmission and the disease is endemic with seasonal fluctuations and outbreaks. It is thought to be of infectious origin, and the mechanism of immune activation may be due to either an antigen or superantigen such as the toxins (see Ch. 16) of Staph. aureus or Strep. pyogenes. A superantigen is a group of proteins that can stimulate many T cells by attaching to part of the T-cell receptor in association with the MHC class II molecules without needing antigen processing.
Treatment, with intravenous immunoglobulin and aspirin reduces the incidence of coronary artery damage and prevents the aneurysms if given early enough.
Viral infections of muscle
Viral myositis, myocarditis and pericarditis
Some viruses, particularly coxsackievirus B, cause myocarditis and myalgia
A cytotoxic effect is seen in animal models after viral attachment to the cellular receptors found in cardiac myocytes and macrophages. Group B, and to a lesser extent group A, coxsackieviruses and certain enteroviruses are the main viral causes of acute myocarditis and pericarditis. There is a slight male predominance in myocarditis and both myocarditis and pericarditis can be mistaken for myocardial infarction, yet the prognosis is good and complete recovery is the rule. There is also evidence for persistent infection linked with chronic myocarditis and chronic dilated cardiomyopathy. The most common cause of viral myocarditis in infants is the coxsackievirus B group, and it may be rapid in onset and fatal. These infections are transmitted by the faecal–oral route and occasionally from pharyngeal secretions. Ingested coxsackieviruses spread from the pharynx or gastrointestinal mucosa to the lymphatics and then to the blood. Invasion of striated muscles, heart or pericardium takes place across small blood vessels and results in acute inflammation. In the heart and pericardium, this gives rise to dyspnoea, pain in the chest, and sometimes mimics a myocardial infarction. Coxsackievirus may be isolated from throat swabs, faecal specimens or occasionally pericardial fluid. Alternatively, viral RNA detection methods may be used, for example in-situ hybridization on endomyocardial biopsy tissue. Mumps and influenza are less common causes of myocarditis or pericarditis. Rubella (see Ch. 23) can cause myocarditis and associated congenital lesions in the fetus.
Group B coxsackieviruses also cause pleurodynia or epidemic myalgia. This condition is sometimes called ‘Bornholm disease’, after the Danish island where there was an extensive outbreak in 1930. There is pain and inflammation involving intercostal or abdominal muscles.
Influenza (especially influenza B in children) can cause pain and tenderness in muscles, but it is not known whether this is associated with viral invasion of muscle. Myalgias are also seen in dengue and in rickettsial and other febrile infections and are probably caused by circulating cytokines.
Laboratory diagnosis in these settings can be difficult, as serology and virus isolation can only give circumstantial evidence for association between that virus infection and a specific organ. Direct detection methods in affected tissue may be more helpful.
The antiviral drug pleconaril has been used to treat enterovirus infections. However, it is no longer in production. There are no specific vaccines for coxsackievirus infections. The mainstay of treatment involves medical management of acute heart failure. This can involve mechanical circulatory support and extracorporeal membrane oxygenation (ECMO) in some clinical situations.
Postviral fatigue syndrome
It has been difficult to establish postviral fatigue syndrome as a clinical entity
The postviral fatigue syndrome or chronic fatigue syndrome is sometimes referred to as myalgic encephalomyelitis, but this is inappropriate because there is no evidence for CNS pathology. It consists of:
• chronic and severe muscle weakness, lasting at least 6 months, often as a sequel to an acute febrile illness
• severe tiredness
• less regularly associated symptoms such as depression, headache and anxiety.
It is more reliably identified when the first two symptoms appear in a previously healthy individual with no history of psychosomatic illness. Several viruses have been suggested as causes. There have been repeated claims for the role of coxsackie B viruses, based on antibody tests and on the detection of a virus-specific protein in the serum of patients, but these results have not been widely confirmed, and the picture remains unclear. A small proportion of cases appear to be due to chronic infection with Epstein–Barr virus (EBV). Occasional reports have associated the condition with HHV-6 as well as other viruses. It has also been suggested that it is due to ‘allergic reactions’ triggered by virus infections.
In 2009, a gammaretrovirus called xenotropic murine leukaemia virus (XMRV) was detected in peripheral blood mononuclear cells from nearly 67% of people with chronic fatigue syndrome compared to 4% of healthy controls. However, the association was not confirmed in other studies and it was thought that the genomic sequences detected were actually part of the enzymes used in the PCR process.
Parasitic infections of muscle
Relatively few protozoan or helminth parasites invade muscle tissues and cause serious disease. Three of the more common are described here to illustrate the variety of organisms and the range of pathology.
Trypanosoma cruzi infection
Trypanosoma cruzi is a protozoan and causes Chagas disease
Chagas disease is also known as American trypanosomiasis (see Ch. 27). The disease is restricted to Central and South America, where it affects an estimated 10–12 million people. It is a zoonosis, and Trypanosoma cruzi has been isolated from more than 150 species of mammal. The parasite is carried by blood-sucking reduviid bugs, which deposit infective trypomastigote stages on to the skin as they defecate while feeding. If these are rubbed into mucous membranes or wounds, the parasites penetrate cells, transform into amastigotes and proliferate. The infected cells then burst, liberating trypomastigotes, and a local lesion is formed. The parasite is dispersed around the body to reinvade other cells. Major sites of infection include the CNS, intestinal myenteric plexus, reticuloendothelial system and cardiac muscle.
Chagas disease is complicated by cardiac conduction disorders, ventricular aneurysm formation or heart failure many years later
Chagas disease may be asymptomatic from the outset or occur as an acute febrile phase, with intense inflammatory changes, followed by a chronic phase that may produce no apparent damage (indeterminate phase), or progress to cause damage years later. In the chronic phase, there is gradual tissue destruction with autoimmune damage playing an important role. The parasite invades the myofibrils of the heart (see Fig. 27.15) causing myocarditis, and muscle fibrils and Purkinje fibres may be replaced by fibrous tissue. As a result of the conduction defects this causes, the heart enlarges, there are cardiac arrhythmias, and heart failure can occur.
Benznidazole or nifurtimox are used to treat the acute phase and some cases in the indeterminate or chronic phase. These drugs are available from the World Health Organization. Posaconazole has been used in individual cases. At time of writing, no vaccine is available, and prevention of infection is the most important measure.
Taenia solium infection
The larval stages of Taenia solium invade body tissues
Tapeworms are intestinal parasites, but the larval stages of several species may invade deeper tissues. The most important of these are:
• Echinococcus granulosus (which causes hydatid disease, see Chs 24 and 28)
• the pork tapeworm Taenia solium.
Humans acquire T. solium infection by eating undercooked infected pig meat in which the cysticercus larvae are found as small, bladder-like structures in the muscle tissue. These larvae are digested out in the intestine and mature into the adult tapeworm, which may reach a length of several metres. T. solium eggs released in human faeces and ingested by a pig hatch in the pig intestine and release larvae which cross the intestinal wall and are carried via the blood to muscle. T. solium is unusual in that its eggs can hatch directly in the human intestine. In areas of poor sanitation, this may result from accidental swallowing of water or food contaminated with eggs. If hatching occurs, larvae can invade and form cysticerci in human muscle or, much more seriously, in the CNS. Cysts in muscle eventually become calcified and can be seen on radiography (Fig. 26.50). Muscle infection is not serious, being largely asymptomatic. Infections are common in many parts of the world, particularly South and Central America and Asia. Avoidance of undercooked pork products is the safest precaution against developing pork tapeworm, whereas good sanitation and good personal hygiene practice are required to avoid ingesting eggs and thus developing cysticercosis.
Figure 26.50 Radiograph showing numerous calcified cysts of Taenia solium in the forearms. (Courtesy of R. Muller and J.R. Baker.)
Trichinella spiralis infection
The larvae of Trichinella spiralis invade striated muscle
This nematode has many unique features. It is able to infect almost any warm-blooded animal, and has a life cycle in which a complete generation (infective stage to infective stage) develops within the body of a single host. Transmission depends upon the ingestion of muscle tissue containing viable infective larvae. As far as humans are concerned, the commonest route of transmission is through infected pig meat, but many other meat sources have been known to transmit infection (e.g. bear, boar, horse). Infections occur worldwide. When infected undercooked meat is eaten, the larvae are digested out in the small intestine and develop rapidly into the adult worms. These live in the mucosa, each female releasing about one thousand newborn larvae directly into the intestinal tissues, from where they are carried in blood or lymph around the body. Eventually, the larvae penetrate striated muscles and mature into the infective stage, transforming muscle cells into a parasite-sustaining nurse cell (see Fig. 28.10).
Light infections are asymptomatic, but the migration and penetration of the larvae is associated with inflammatory reactions, which can be severe and life-threatening when a person is heavily infected. Various symptoms are associated with this phase, of which fever, muscle pains, weakness and eosinophilia are characteristic. Myocarditis may also occur, although the parasite does not develop in the heart.
Diagnosis by symptoms usually occurs after the parasites have invaded the muscles and treatment then is difficult. Albendazole or mebendazole are used. Adjunctive corticosteroids are given in severely symptomatic cases.
Sarcocystis
Sarcocystis is a rare muscle parasite
The cyst stages of Sarcocystis, a protozoan related to Toxoplasma, are occasionally reported in human muscles.
Joint and bone infections
Joints and bones will be considered separately for convenience, but joint lesions often spread to involve neighbouring bone, and vice versa (e.g. in tuberculosis).
Reactive arthritis, arthralgia and septic arthritis
Arthralgia and arthritis occur in a variety of infections and are often immunologically mediated
Examples of such infections are outlined in Table 26.6. Joints can become infected by the haematogenous route or directly following trauma or surgery, but in many cases the condition is immunologically mediated rather than due to microbial invasion of the joint. The microbe responsible is at a distant site in the body and causes a ‘reactive arthritis’. Reactive arthritis and arthralgia occur after certain enteric bacterial infections, and the arthralgia in rubella and hepatitis B infections is of similar origin. In this type of arthritis, more than one joint is usually affected.
Table 26.6 Arthralgia and arthritis in infectious diseases
|
Infectious agent |
Comments |
|
Viral arthritis |
|
|
Hepatitis B |
Occurs in prodromal period; due to circulating immune complexes |
|
Rubella |
Especially in young women, often follows live virus vaccine |
|
Mumps |
Unusual; mostly in men |
|
Ross River and other togaviruses |
Mosquito-transmitted infections in Australia (Ross River) and Africa |
|
Parvovirus |
May follow adult infection |
|
Reactive arthritis |
|
|
Campylobacter, Yersinia, salmonellae, shigellae, Chlamydia trachomatis (Reiter’s syndromea) |
‘Post-infectious’ arthritis, HLA B27-associated, no bacterial invasion of joint, immune mediated |
|
Septic arthritis |
|
|
Staphylococcus aureus |
Commonest cause of suppurative arthritis |
|
Streptococci (Group A and B) |
Common in adults and children |
|
Haemophilus influenzae |
Occurrence in children has decreased with H. influenzae vaccine |
|
Neisseria gonorrhoeae |
May affect multiple joints |
|
Mycobacterium tuberculosis |
Often with bone lesions, especially weight bearing joints and bones |
|
Borrelia burgdorferi |
Arthritis a late feature of Lyme disease |
|
Gram-negative bacilli |
Neonates, the elderly, patients with immune deficiency disorders |
|
Sporothrix schenckii |
Fungal infection of joints; increased risk with HIV infection |
a Urethritis, arthritis, uveitis, mucocutaneous lesions; complicates a small percentage of cases of chlamydial urethritis.
Ankylosing spondylitis is associated with Klebsiella infection, and it has been suggested that the antigenic similarity between Klebsiella and HLA B27 antigens provokes a cross-reactive immune response that causes the disease. So far, there is no evidence that rheumatoid arthritis is caused by either viruses or other microbes.
Circulating bacteria sometimes localize in joints, especially following trauma
Such bacterial localization can then cause a suppurative (septic) arthritis. Generally, a single joint is involved. Joints are very susceptible, particularly if they are already damaged, for instance by rheumatoid arthritis, or if a prosthesis has been inserted. Knees are most commonly affected, followed by hips, ankles (see Fig. 21.5B) and elbows. Signs include a fever, joint pain, limitation of movement and swelling, and usually a joint effusion. Bacteria can be isolated from the joint fluid or seen in the centrifuged deposit, and the commonest organism is Staph. aureus. Sometimes the source of the circulating bacteria is obvious (e.g. a septic skin lesion), but often no source is apparent.
Osteomyelitis
Bone can become infected by adjacent infection or haematogenously
As with joints, infection of bones can be by the direct route (e.g. from a nearby focus of infection, after fractures, after orthopaedic surgery) or from circulating microbes. The commonest cause of haematogenous osteomyelitis is Staph. aureus, but when infection is from a neighbouring site it is generally mixed, with Gram-negative rods and occasionally anaerobes also present. There seems to be no equivalent to reactive arthritis, in which inflammation is due to infection at a distant site.
Acute osteomyelitis typically involves the growing end of a long bone, where sprouting capillary loops adjacent to epiphyseal growth plates promote the localization of circulating bacteria. It therefore tends to be a disease of children and adolescents, and may follow non-penetrating injury to the bone.
Osteomyelitis results in a painful tender bone lesion and a general febrile illness.
Osteomyelitis is treated with antibiotics and sometimes surgery
The infection is diagnosed from blood cultures taken before the start of antimicrobial therapy or, if there is an open lesion, from a bone biopsy. Periosteal reaction and bone loss may be visible radiologically (Fig. 26.51). Treatment is begun on a ‘most likely’ basis (e.g. nafcillin for MSSA, see above) as soon as microbiologic samples have been taken.
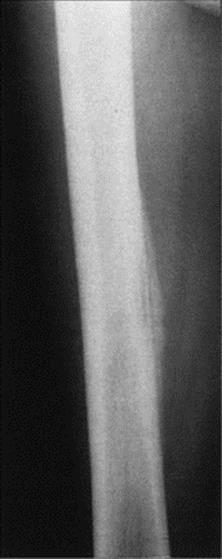
Figure 26.51 Acute staphylococcal osteomyelitis in the femur of a 24-year-old woman. There is a well-defined periosteal reaction in relation to the midshaft of the femur and an underlying lucency.
(Courtesy of A.M. Davies.)
Osteomyelitis can become chronic, especially when there are necrotic bone fragments to act as a continued source of infection. Surgical intervention for debridement and drainage, as well as prolonged courses of antibiotics, may be necessary.
Tuberculosis may affect the spine, the hip, the knee and the bones of the hands and feet, and in resource-rich countries is particularly seen in immigrants from the Indian subcontinent. Constitutional disturbances are often absent, but the site is generally painful and pressure from a tuberculous abscess in the spine can cause paraplegia.
Infections of the haemopoietic system
Many infectious agents cause changes in circulating blood cells
Examples of such agents include:
• Bordetella pertussis, which causes lymphocytosis
• EBV and cytomegalovirus infections, which cause mononucleosis
• Plasmodium spp. which cause anaemia and thrombocytopenia.
A smaller number of infectious agents act directly on cells in the bone marrow (human parvovirus) or cause malignant transformation of lymphocytes, for example, human T-cell lymphotropic virus (HTLV) type 1. The range of possibilities is summarized in Table 26.7. HTLV-1 and HTLV-2 are mentioned in Chapters 21 and 24, but are described more fully below.
Table 26.7 Examples of microbes affecting blood cells or haemopoiesis
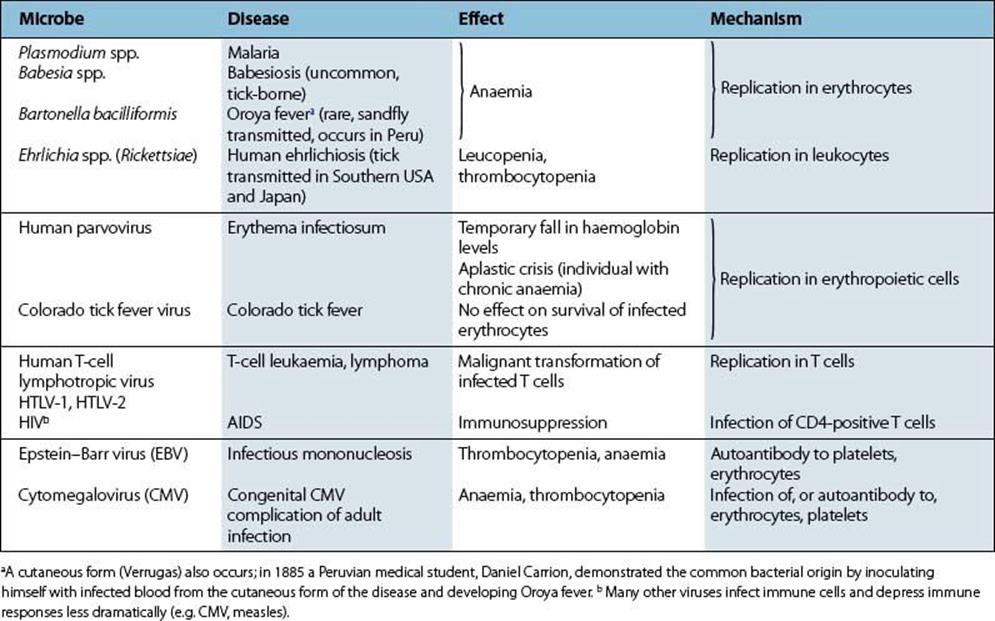
Human T-lymphotropic virus-1 infection
Human T-lymphotropic virus -1 is mainly transmitted by maternal milk
Human T-lymphotropic virus -1 was first isolated in 1980 from a patient with adult T-cell leukaemia (ATLL). Infection is widespread, especially in certain islands in the West Indies and Japan, where 5–15% of the population are infected, and also in South America and parts of Africa. Transmission is primarily via maternal milk, less effectively via sexual intercourse, and by blood-contaminated equipment in injecting drug users.
Human T-lymphotropic virus -1 infects T cells, and up to 5% of those infected develop T-cell leukaemia
Human T-lymphotropic virus-1 infects T cells and persists. The tax gene product, a transcriptional activator protein, stimulates transcription of host genes controlling production of interleukin 2 (IL-2), IL-2 receptor and other molecules, thus affecting cell replication. Infected T cells proliferate, and if in addition there are certain chromosomal abnormalities, malignant transformation takes place.
Clinically, the patient develops a mild febrile disease with lymphadenopathy. The skin is often involved, with nodule and plaque formation, and pleural effusion or aseptic meningitis can occur. There is also increased susceptibility to opportunist infections such as Pneumocystis jirovecii andStrongyloides stercoralis. Depressed delayed hypersensitivity responses to tuberculin are associated. Polymyositis has been described. Up to 5% of infected individuals eventually develop T-cell leukaemia, which has a high and rapid mortality rate, and a similar proportion progress to ‘tropical’ spastic paraparesis (TSP), also known as HTLV-associated myelopathy (HAM), in which there is primary demyelination (see Ch. 24). Neural cells do not appear to be infected, and it is not known how the virus causes a neurologic disease.
Detection of HTLV-1 and HTLV-2-specific antibody is based on serological methods with type differentiation by immunoblot. Antiretroviral agents other than protease inhibitors have been shown to inhibit viral replication and are under investigation as part of the management of individuals with ATLL or TSP. Other treatments have been examined with limited success. HTLV infection can be transmitted by HTLV antibody-positive individuals and they should not donate blood or organs. HTLV antibody screening of blood donors is now included in many countries.
Human T-lymphotropic virus-2 infection
Human T-lymphotropic virus-2 was first isolated in 1982 from a patient with T-cell hairy leukaemia, although it is not the usual cause of this condition. HTLV-2 is closely related to HTLV-1, is transmitted by similar routes and has been reported in injecting drug users and native Amerindian tribes in North, Central and South America. It has been associated with a number of neurological conditions including occasional reports of myelopathy.
![]()
 Key Facts
Key Facts
• The intact skin is an invaluable barrier that defends the body against invasion.
• A wide range of organisms is associated with skin infection and disease.
• Bacteria, fungi and viruses usually gain access through breaches of the barrier caused by trauma.
• Some parasites initiate their own penetration into the skin (hookworm, schistosomes).
• Other microbes are introduced into the skin by arthropod vectors.
• Once in the skin, microbes cause local infections or disseminate through the body to distant sites.
• Pathogens may be acquired by other routes, disseminate in the body and then localize in the skin or cause toxic or immunopathologic manifestations in the skin.
• Superficial infections of the skin are among the commonest human infections (boils, impetigo, warts, acne, ringworm).
• Invasion of pathogens deeper into dermal and subdermal tissues may produce severe infections that can be rapidly fatal, as in gangrene, or slow but progressive deformation and destruction, as in leprosy.
• Infections of muscle usually arise from invasion from the outside, whereas infections of joints are more often blood-borne.
• Bone infections may arise either by local spread from an infected joint or as a result of haematogenous seeding.
• Bone marrow cells or leukocytes may be invaded by viruses that interfere with haemopoiesis (parvovirus), cause malignant transformation (HTLV-1), or interfere with the immune system (EBV, HIV).
![]()
